CHARLOTTE, N.C., April 15, 2013 (GLOBE NEWSWIRE) -- Phenogen Sciences, Inc. recently presented study results demonstrating that BREVAGen™, a first-in-class, scientifically-validated predictive breast cancer risk assessment test, more accurately identifies a woman's chances of getting breast cancer than the Breast Cancer Risk Assessment Tool (BCRAT) alone, leading to increased patient compliance with performing regular self breast exams and annual screenings. The clinical study, Impact of Genomics on the Assessment and Management of Breast Cancer Risk in a Woman's Healthcare Clinic was performed at Personalized Women's Healthcare in Plano, TX and the findings were presented at the 23rd Annual National Interdisciplinary Breast Center Conference in Las Vegas, NV, March 23-27, 2013.
Typically, the identification of patients who are at an elevated risk for sporadic (non-hereditary, non-familial) breast cancer is performed using the National Cancer Institute's (NCI) BCRAT. But recently, with the introduction of the BREVAGen test, a woman's risk for developing sporadic, estrogen-positive breast cancer is more accurately identified. BREVAGen examines a woman's clinical risk factors, such as their lifetime exposure to estrogen, combined with scientifically validated markers to determine each patient's personalized five-year and lifetime risk of developing breast cancer. 1 BREVAGen test results support current American Cancer Society (ACS), American Society of Clinical Oncology (ASCO) and The National Comprehensive Cancer Network (NCCN) guidelines for prevention and early detection of breast cancer.2,3
"Our practice has been using the BREVAGen test for over a year and it has helped us to make more informed decisions in how we monitor our patients who are at risk and develop personalized breast health plans," said Eric Jacoby, M.D. "We are pleased that the results of our report clearly demonstrate that genetic information provided by the BREVAGen test show significant reductions in the five year and lifetime intermediate risk groups, 35 percent and 23 percent respectively."
Study Results
In the study of 197 women over the age of 35, 68 percent had a higher than the population average five year risk for developing breast cancer, as assessed by the NCI-BCRAT. Evidence suggests that patients who are close to the 1.66 percent ASCO threshold, where it is important that an accurate risk assessment is made, are those who may see an impact of the BREVAGen test. This intermediate risk group in the BREVAGen study, who categorically fell between 1.5 and 2.0 percent, saw their five-year risk reduced by 35 percent. In the case of the lifetime risk where the ACS classifies the threshold at 20 percent, BREVAGen provided a 23 percent reduction for the intermediate risk group. The study results are clearly aligned with the ASCO and ACS thresholds and showed that when adding genotypic information from the BREVAGen test to the BCRAT, there is a significant reclassification of individual risk. Alignment with the ASCO and ACS thresholds enables healthcare professionals to develop future health plans for their patients.
How BREVAGen Works
The BREVAGen predictive risk test is administered in a physician's office using a simple, non-invasive "oral-swab". Following analysis in our CLIA-certified laboratory, physicians receive a comprehensive genetic risk prediction report to review with the patient. The patient's risk of breast cancer is calculated by combining their relative risk score from seven genetic markers, called SNP's (single nucleotide polymorphisms), with factors that comprise the patient's clinical and reproductive history including current age, age at menarche, age at live first birth and race/ethnicity.
The BREVAGen test provides five-year and lifetime predictive risk assessments to more accurately evaluate the patient's risk of developing sporadic breast cancer, regardless of family history.
Clinically Validated
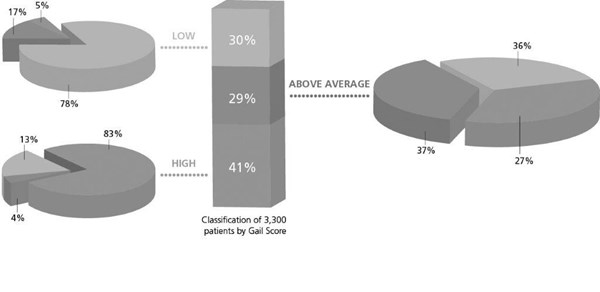
BREVAGen was proven superior in determining breast cancer risk compared to Gail score alone.1 BREVAGen is the first genetic risk prediction test to have been validated in a large scale, peer reviewed, case controlled study. Utilizing data from the U.S. Women's Health Initiative (WHI) Clinical Trial, 3,300 women underwent breast cancer assessment utilizing the BREVAGen test. Of those 3,300 women, 1,664 were diagnosed with breast cancer and 1,636 were in the breast cancer-free control group.
About Breast Cancer
• Approximately one in eight women will get breast cancer equating to approx. 207,000 American women.4
• Up to 80% of women who get breast cancer do not have a strong family history of the disease.2
• Risk of invasive vs. non-invasive breast cancer is approximately four times greater in women age 40-645
• Approximately 75% of all breast cancer is estrogen-receptor positive and, if detected early, can be effectively treated with five-year survival rates of over 95%
Breast Cancer Demographics
According to the American Cancer Society, breast cancer is the most common cancer among women in the United States, other than skin cancer. It is the second leading cause of death in women, after lung cancer.4 According to the 2010 breast cancer demographics there are:
• Approximately 207,000 new cases diagnosed each year4
• Approximately 40,000 deaths related to breast cancer in women, annually4
BREVAGen reclassified 64% of above average Gail risk subjects (see attached chart)1
BREVAGen reclassified 64% of Gail scores above average risk subjects as either high or low risk for development of hormone-dependent breast cancer. Furthermore, the BREVAGen test reclassified the breast cancer risk for 33% of the total 3,300 trial subjects.1
About BREVAGen
The first product in Phenogen Sciences' portfolio, BREVAGen is a predictive risk test for sporadic, hormone-dependent breast cancer. The BREVAGen test combines a woman's clinical history of estrogen exposure with the presence of identified genetic markers to determine her five-year and lifetime risk of developing breast cancer. For women whose clinical profile indicates prolonged estrogen exposure, BREVAGen helps to provide a more accurate risk assessment for estrogen-receptor positive breast cancer. The test results assist physicians in developing a personalized care path toward managing each woman's risk of developing breast cancer with greater precision than ever before. For more information, visit http://www.brevagen.com.
• Non-invasive, easy-to-use predictive risk assessment test
• The first genetic risk prediction test to have been validated in a large-scale, peer-reviewed, case-controlled study1
• BREVAGen reclassified 64% of subjects with an above average Gail risk score as either high or average risk for development of breast cancer1
• Aligns with existing industry guidelines for the prevention of estrogen-receptor positive breast cancer2,3
Click here for BREVAGen B-roll/video:
• Video One: http://www.youtube.com/watch?v=-em2pNeOMNs &feature=plcp
• Video Two: http://www.youtube.com/watch?v=BSExN7mNlzA &feature=plcp
About Phenogen Sciences, Inc.
Phenogen Sciences, the U.S. subsidiary of Australia-based Genetic Technologies Limited, is a pioneer in personalized healthcare. Phenogen Sciences offers novel predictive testing and assessment tools that help physicians proactively manage women's health risks. Phenogen Sciences' lead product, BREVAGen™ is a scientifically validated test that combines a woman's clinical history of estrogen exposure with her genetic predisposition to its effects; more accurately categorizing her personal risk of developing breast cancer. For more information, visit http://www.phenogensciences.com.
About Genetic Technologies Limited
Genetic Technologies is an established diagnostics company with more than 20 years of experience in commercializing genetic testing, non-coding DNA and product patenting. The company has operations in Australia and the U.S. and is dual-listed on the ASX (GTG.AX) and NASDAQ (GENE). Genetic Technologies is focused on the commercialization of its patent portfolio through an active out-licensing program and the global expansion of its oncology and cancer management diagnostics assets. For more information, please visit http://www.gtglabs.com.
1 Mealiffe M, Stokowski RP, Rhees, BK, et al. J Nat Cancer Inst. 2010;102(21):1618-1627.
2 Saslow D, Boetes C, Burke W, et al. CA Cancer J Clin. 2007;57(2):75-89.
3 Visvanathan K, Chlebowski RT, Hurley P, et al. J Clin Oncol. 2009;27(19):3235-3258.
4 Breast Cancer Overview. American Cancer Society. Accessed 3/14/12 at
http://www.cancer.org/Cancer/BreastCancer/OverviewGuide/breast-cancer-overview?docSelected=breast-cancer-
overview-key-statistics
5 DeSantis C, Siegel R, Bandi P, Jemal A. CA: A Cancer Journal For Clinicians. 2011;61: 409-418
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=18124