Dublin, Nov. 06, 2023 (GLOBE NEWSWIRE) -- The "Edema Clinical Trials Market Size, Share & Trends Analysis Report By Phase (Phase I, Phase II, Phase III, Phase IV), By Participant (Pediatrics, Adults, Geriatrics), By Sponsor, By Type, By Region, And Segment Forecasts, 2023 - 2030" report has been added to ResearchAndMarkets.com's offering.
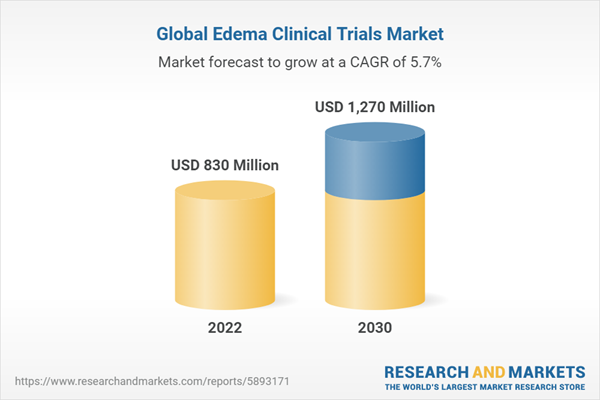
The global edema clinical trials market size is expected to reach USD 1.27 billion by 2030, registering a CAGR of 5.71%
The edema-related disorders and conditions market is being propelled by several factors, including the increasing prevalence of edema-related health issues, ongoing advancements in medical research and technology, and the growing support and incentives from regulatory bodies for conducting clinical trials. Various governments and health authorities worldwide have implemented policies aimed at expediting the approval process and facilitating research, particularly in areas with unmet medical needs like edema.
The COVID-19 pandemic had a profound impact on the clinical trials market, disrupting research endeavors and reshaping the landscape of medical research. The pandemic led to an urgent allocation of healthcare resources to combat the virus, resulting in the cancellation of numerous ongoing clinical trials, including those focusing on edema-related conditions. Additionally, resources and funding were diverted away from other medical areas towards COVID-19 research, creating challenges for researchers seeking support to continue their studies.
Despite these challenges, market players have undertaken various initiatives and product launches that have contributed to market growth. For example, Eyebiotech Limited (EyeBio) initiated Phase 1b/2 clinical trials for Restoret in patients with diabetic macular edema and neovascular age-related macular degeneration in June 2023. Similarly, in August 2021, Novartis reported favorable outcomes from Phase III clinical trials of Beovu in diabetic macular edema, including dosing intervals of 16 weeks.
Among clinical trial phases, the Phase II segment led the market in 2022, accounting for approximately 35.71% of the market share. Phase II trials play a critical role in assessing the safety and preliminary efficacy of new treatments for edema-related conditions.
In terms of patient demographics, the adult segment dominated the market in 2022. This growth is attributed to the higher likelihood of adults developing underlying medical conditions such as heart failure, kidney disease, and venous insufficiency, all of which can contribute to the development of edema.
Pharmaceutical companies held the largest market share in 2022, owing to their extensive research and development capabilities, including access to state-of-the-art laboratories and specialized expertise in drug development.
Among the various types of edema, localized edema accounted for the largest market share in 2022. This can be attributed to the increasing prevalence of localized edema-related conditions, the presence of unmet medical needs, and the availability of research funding.
North America dominated the market in 2022, representing approximately 41.44% of the total market share. This is due to the region's advanced healthcare infrastructure, the presence of numerous leading pharmaceutical companies, and a supportive regulatory environment.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 175 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value (USD) in 2022 | $830 Million |
| Forecasted Market Value (USD) by 2030 | $1270 Million |
| Compound Annual Growth Rate | 5.7% |
| Regions Covered | Global |
Company Profiles
- Otsuka Holdings
- Hoffmann-La Roche
- Novartis Pharmaceuticals
- Bayer
- Genentech, Inc
- Biogen
- Johnson & Johnson
- Merck & Co., Inc.
- Sanofi S.A.
- AstraZeneca plc
- Bristol Myers Squibb Company
- GlaxoSmithKline plc
- AbbVie Inc.
Key Topics Covered:
Chapter 1. Methodology and Scope
Chapter 2. Executive Summary
2.1. Market Snapshot
2.2. Segment Snapshot
2.3. Competitive Landscape Snapshot
Chapter 3. Market Variables, Trends, & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Trends and Outlook
3.3. Market Dynamics
3.3.1. Market Driver Analysis
3.3.2. Market Restraint Analysis
3.4. Business Environment Analysis
3.4.1. PESTLE Analysis
3.4.2. Porter's Five Forces Analysis
3.5. COVID-19 Impact Analysis
Chapter 4. Phase Business Analysis
4.1. Edema Clinical Trials Market: Phase Movement Analysis, 2022 & 2030
4.2. Phase I
4.2.1. Phase I Market, 2018 - 2030 (USD Million)
4.3. Phase II
4.4. Phase III
4.5. Phase IV
Chapter 5. Participant Business Analysis
5.1. Edema Clinical Trials Market: Participant Movement Analysis, 2022 & 2030
5.2. Pediatrics
5.2.1. Pediatric Market, 2018 - 2030 (USD Million)
5.3. Adults
5.4. Geriatrics
Chapter 6. Sponsor Business Analysis
6.1. Edema Clinical Trials Market: Sponsor Movement Analysis, 2022 & 2030
6.2. Pharmaceutical Companies
6.2.1. Pharmaceutical Companies Market, 2018 - 2030 (USD Million)
6.3. Academic Institutions
6.4. Government Bodies
Chapter 7. Type Business Analysis
7.1. Edema Clinical Trials Market: Type Movement Analysis, 2022 & 2030
7.2. Systemic Edema
7.2.1. Systemic Edema Market, 2018 - 2030 (USD Million)
7.3. Localized Edema
Chapter 8. Regional Business Analysis
8.1. Regional Market Snapshot
Chapter 9. Competitive Landscape
9.1. Company Categorization
9.2. Strategy Mapping
9.2.1. Acquisition
9.2.2. Collaborations
9.2.3. New Platform Launches
For more information about this report visit https://www.researchandmarkets.com/r/r3zy2a
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
