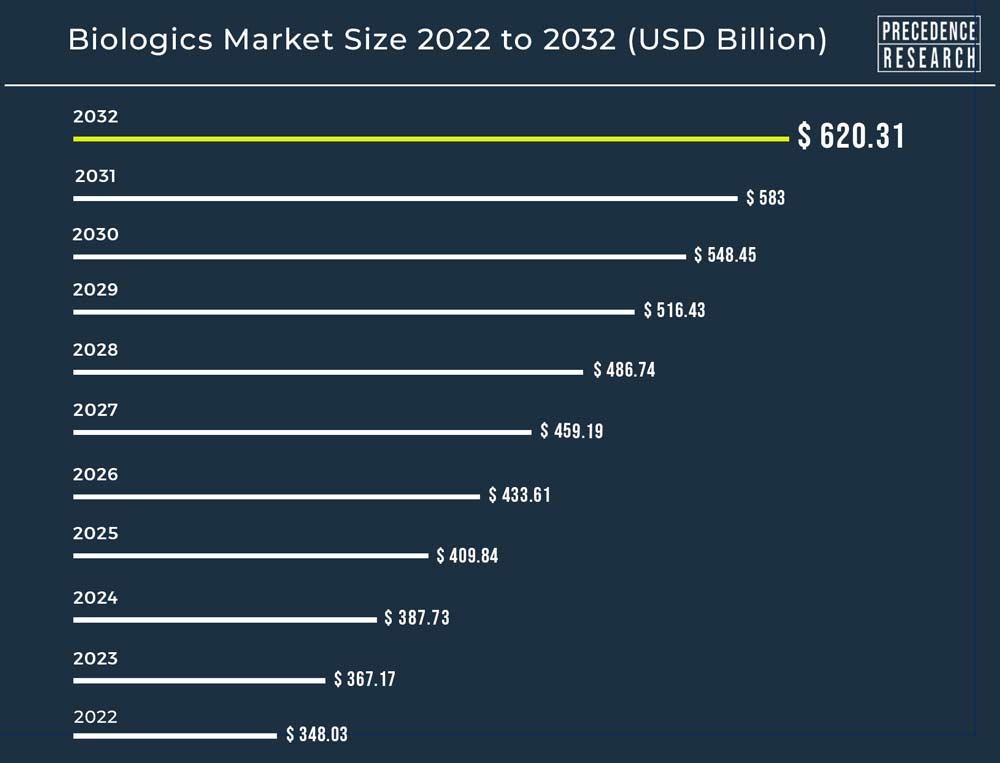
Ottawa, Jan. 08, 2024 (GLOBE NEWSWIRE) -- The global biologics market size accounted for USD 387.73 billion in 2024, grew to USD 409.84 billion in 2025 and the industry is expanding at a CAGR of 6% from 2023 to 2032. Europe Region holds the largest market share of 34% of the biologics market during the forecast period.
The biologics market is driven by the increasing number of chronic illnesses impacting millions of individuals globally and placing a heavy strain on healthcare systems. Biologics provide a more focused and efficient therapy alternative for many diseases, boosting demand and propelling market expansion.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/1638
Medications classified as biologics are made from living things or their byproducts. This indicates that they differ from conventional medicines, generally created using lab-synthesized tiny molecules. Proteins, nucleic acids, cells, tissues, and other components can all be used to develop biologics, which are frequently more extensive and complicated. Although biologics functions in many different ways, they frequently target particular chemicals or bodily cells. Because of this, they may be more effective than conventional medications, which frequently have broader effects. Biologics, however, could have effects and cost more money. The field of biologics is expanding quickly in medicine, and new kinds of biologics are created regularly.
- The FDA put a clinical hold on the trial assessing the innovative medication, LN-145 TIL, for those with non-small cell lung cancer (NSCLC), according to a press release from the drug's producer, Iovance Biotherapeutics, dated January 20, 2024. An enrolled patient passed away, possibly because of the non-myeloablative lymphodepletion pre-conditioning regimen. This led to the decision to halt the IOV-LUN-202 trial. In individuals with unresectable or metastatic NSCLC that
- worsened (became worse or ceased responding) during or following chemotherapy & an anti-PD-1 immunotherapy treatment, IOV-LUN-202 assesses LN-145 TIL.
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
Regional Snapshot
North America is expected to have most of the biologics market share over the forecast period. The U.S. and Canada have state-of-the-art research facilities and healthcare infrastructure supporting innovation and growth in the biologics industry. North American regulatory agencies, including Health Canada and the U.S. Food and Drug Administration (FDA), have established precise and well-defined procedures for approving biologics. This regulatory support spends heavily on the research and development of biologics and makes it easier for novel biologics to be introduced to the market on time. It frequently works in alliances and partnerships with foreign biotech companies, research groups, and academic institutions. These partnerships improve the total research capacity.
For instance, Canada and Alberta signed a bilateral agreement worth over $1 billion to enhance healthcare over three years. Through this investment, more people will have access to primary healthcare providers, mental health services will be available sooner, and health data will be more easily accessible.
- In December 2023, Casgevy and Lyfgenia, two groundbreaking medications that constitute the initial cell-based gene therapies for treating sickle cell disease (SCD) in individuals 12 years of age and older, have been cleared by the U.S. Food and Drug Administration. Moreover, one of these treatments, Casgevy, represents a groundbreaking development in gene therapy as the first FDA-approved medication to use cutting-edge genome editing technology.
- The Department of Health and Human Services is going to spend $40 million to expand the function of biological manufacturing for active pharmaceutical ingredients (APIs), antibiotics, and the crucial starting materials required to produce vital drugs and react to pandemics based on the summit on biotechnology and biomanufacturing in September 2022.
Biologics Market Report Scope:
| Report Coverage | Details | |
| CAGR 2023-2032 | 6 | % |
| Global Market Size in 2023 | USD 367.17 Billion | |
| Global Market Size by 2032 | USD 620.31 Billion | |
| Base Year | 2022 | |
| Forecast Period | 2023 to 2032 | |
| Largest Market | Europe | |
| Segments Covered | By Source, By Product, By Indication, By Manufacturing, By Distribution Channel, and By Geography | |
We've prepared a service to help you write your own Go-To-Market strategy.
Click to Unlock Your GTM Strategy for the Biologics Market
Highlights of Biologics Market
Source Insights
The microbial segment is expected to dominate the biologics market share over the forecast period. Microbial-based biologics have benefits in terms of cost-effectiveness and production scalability. Examples of these include recombinant proteins made from genetically modified bacteria or yeast. Microbial systems are typically more effective than mammalian cell cultures when producing vast amounts of biologics.
The development of genetic engineering techniques has also made it easier to optimize microbial strains for improved protein expression, purification, and general bioprocess efficiency. As a result, the biologics market is now more competitive, and production costs are lower.
Product Insights
The monoclonal antibodies segment is expected to dominate the market during the forecast period. The segment expansion is attributed as therapy outcomes are highly selective because monoclonal antibodies are specifically designed to target antigens. This accuracy increases the effectiveness of treatment and reduces off-target effects. It aligns with customized medicine, which customizes care for each patient according to their unique genetic and biochemical profiles. This strategy reduces unfavorable reactions while improving treatment results.
Additionally, they have helped treat several illnesses, such as infectious diseases, autoimmune disorders, and cancer. Their broad popularity is partly due to their adaptability and efficacy in various treatment domains.
Personalized your customization here: https://www.precedenceresearch.com/customization/1638
Manufacturing Insights
The in-house segment is expected to dominate the biologics market share over the forecast period. Businesses favour internal production of biologics, like recombinant proteins and monoclonal antibodies, to maintain better control over the manufacturing process, guarantee product quality, and safeguard intellectual property. Research, growth, and manufacturing teams can communicate more easily when production is done in-house, accelerating and improving production schedules. Furthermore, bioprocessing methods and technological developments make internal production more practical and economical.
For instance, South Korea's advantageous location in Asia makes it desirable for biologics contract production. The government also actively supports and encourages this, which makes South Korea a global leader in sophisticated manufacturing. The US-based West Pharmaceutical Services, Inc. is among the numerous participants taking advantage of this chance. West sees tremendous opportunities in the South Korean market as local pharmaceutical businesses increase their capacity and sharpen their skills in contract manufacturing, biologics research and development, and other areas.
Indication Insights
The oncology segment is expected to capture most of the market share over the forecast period. This market is attractive due to the rising incidence of cancer and the expanding understanding of biologics as viable therapeutic alternatives. Complex compounds called biologics, generated from living things, provide targeted and individualized cancer therapy strategies. Compared to conventional treatments, these medications frequently have fewer adverse effects and a higher specificity.
The American Cancer Society estimates the number of new cases and fatalities from cancer in the US. The United States is expected to see 609,820 cancer deaths and 1,958,310 new cancer cases in 2023, according to data gathered by the National Center for Health Statistics.
A growing body of knowledge on the molecular causes of cancer has led to the development of biologics that target cancer cells or the environment surrounding tumours. Patient outcomes are enhanced by this focused strategy, which maximizes therapy efficacy while reducing harm to healthy cells. Biologics have become increasingly important in oncology due mainly to the growing use of immunotherapy, a subgroup of biologics, in cancer treatment. Monoclonal antibodies and inhibitors of checkpoints are examples of immunotherapeutic drugs that use the body's immune system to eliminate cancer cells. These agents have shown encouraging results in treating a variety of cancers.
Market Dynamics
Driver: Emergence of precision medicine and personalized treatment options
Due to developments in genetic and molecular testing, healthcare practitioners are becoming more adept at identifying patients with specific genetic abnormalities or biomarkers that increase their susceptibility to particular diseases or increase their likelihood of responding to specific therapies. As a result, targeted biologics which target these characteristics have been developed. These treatments have the potential to be less hazardous and more successful than conventional, one-size-fits-all pharmaceuticals.
Restraint: High cost of production and development
Biologics are complex compounds that are frequently produced from live cells. Examples of these include genetically modified proteins and antibodies. Because of this, producing them is more expensive and complicated than producing conventional synthetic medications. It takes capital to set up and maintain facilities and equipment for biomanufacturing, which calls for specialized knowledge and technology. Finding and characterizing the particular cell lines or organisms required for biologic production can also be expensive and time-consuming. Finally, downstream processing and clinical trials add to the expense of development. This high-cost barrier may restrict a patient's access to these life-saving treatments, especially in underdeveloped nations. Additionally, it poses difficulties for smaller biopharmaceutical businesses and may impede industry innovation.
Opportunity: Development of personalized biologics for cancer treatment
Conventional cancer therapies, such as radiation therapy and chemotherapy, can have significant adverse effects and are frequently ineffective. Personalized biologics, on the other hand, are made to specifically target the genetic abnormalities that are causing a patient's cancer. Therefore, they are far less harmful and more effective than conventional therapies. The invention of new technologies for providing biologics to cancer cells, the growing awareness of the advantages of personalized medicine, and the increasing affordability of genomic testing, which may determine the genetic mutations leading to a patient's cancer, are some of the factors propelling the growth of this market.
Browse More Insights:
- Plasma Protein Therapeutics Market: The global plasma protein therapeutics market size reached USD 28.13 billion in 2023 and is expected to hit around USD 44.01 billion by 2032, poised to grow at a CAGR of 5.10% during the forecast period from 2023 to 2032.
- Surgical Procedures Market: The global surgical procedures market size reached USD 3.15 trillion in 2023 and is projected to hit around USD 5.10 trillion by 2032, registering a CAGR of 5.50% during the forecast period from 2023 to 2032.
- Telerehabilitation Market: The global telerehabilitation market size was estimated at USD 3.58 billion in 2023 and is projected to hit around USD 12.90 billion by 2032, registering a CAGR of 15.30% during the forecast period from 2023 to 2032.
- Wireless Implants Market: The global wireless implants market size was estimated at USD 6.70 billion in 2023 and is projected to hit around USD 29.51 billion by 2032, registering a CAGR of 17.90% during the forecast period from 2023 to 2032.
- Wound Care Market: The global wound care market size surpassed USD 23.57 billion in 2023 and is projected to hit around USD 35.33 billion by 2032, registering a CAGR of 4.40% during the forecast period from 2023 to 2032.
Recent Developments
- In December 2023, the fully integrated worldwide biosimilars business Biocon Biologics Ltd. (BBL), a subsidiary of Biocon Ltd., and Sandoz signed a distribution agreement that gives the former the sole right to sell, market, and distribute “Adalimumab BS for injections under the skin [FKB]” in Japan.
- In December 2023, Leading Brazilian agricultural biologicals business, Agrivalle recently announced a new collaboration with Ginkgo Bioworks, the firm creating the industry-leading platform for biosecurity and cell programming. Together, the companies will develop state-of-the-art technologies that propel Agrivalle's biological products forward, such as biocontrol agents and next-generation fertilizers.
- In December 2023, Cytovance Biologics partnered strategically with Alcami Corporation, a fellow CDMO, to enhance its large molecule biologic production capacity and sterile fill finish product line.
Market Key Players
- Eli Lilly & Company
- Sanofi
- Pfizer Inc.
- Merck & Co. Inc
- Novo Nordisk A/S
- Samsung Biologics
- F Hoffman La Roche
- Celltrion Addgene
- Amgen
- Abbvie Inc.
Market Segmentation
By Source
- Microbial
- Mammalian
- Others
By Product
- Monoclonal Antibodies
- Human mABs
- Humanized mABs
- Chimeric mABs
- Murine mABs
- Vaccines
- Recombinant Proteins
- Antisense, RNAi & molecular therapy
- Cell Based Therapies
- Stem Cell Therapy
- CAR-T Cell Therapy
- Tissue Engineering
- Others
By Indication
- Oncology
- Immunological Disorders
- Cardiovascular Disorders
- Hematological Disorders
- Others
By Manufacturing
- Outsourced
- In-house
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa (MEA)
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/1638
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
Unlocking Market Insights through Data Excellence
The "Precedence Statistics" flexible dashboard is a powerful tool that offers real-time news updates, economic and market forecasts, and customizable reports. It can be configured to support a wide range of analysis styles and strategic planning needs. This tool empowers users to stay informed and make data-driven decisions in various scenarios, making it a valuable asset for businesses and professionals looking to stay ahead in today's dynamic and data-driven world.
To Access our Premium Real Time Data Intelligence Tool, Visit: www.precedencestatistics.com
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com
Our Blogs:
https://www.towardshealthcare.com
https://www.towardspackaging.com
For Latest Update Follow Us:
