Dublin, March 20, 2024 (GLOBE NEWSWIRE) -- The "Clinical Trial Supplies Global Market Report 2024" report has been added to ResearchAndMarkets.com's offering.
The specialized field of clinical trial supplies is experiencing a significant upturn, echoed by the remarkable growth forecasted for the Asia-Pacific region. This report, offering a comprehensive analysis of the global clinical trial supplies market through to 2028, underscores the integral role of the clinical trial infrastructure in the evolution of medical research and patient care.
The study identifies several pivotal elements contributing to the expansion of the clinical trial supplies industry. Innovations within the market are largely influenced by the rise in chronic diseases, complex regulatory landscapes, increased clinical trial activity for novel therapies, and an emphasis on patient-centric approaches.
Key Insights Highlighted in the Report:
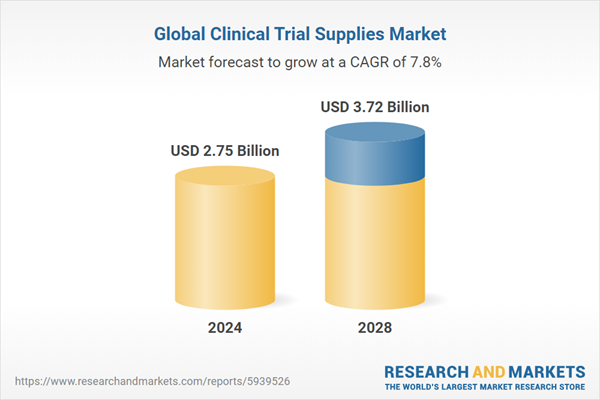
- Growth statistics showcasing a surge from $2.52 billion to $3.72 billion from 2023 to 2028, marking a CAGR of 7.8%.
- Strategic acquisitions, such as the extensive $17.4 billion acquisition of PPD Inc. by Thermo Fisher Scientific Inc., aiming to enhance clinical trial service capabilities.
- Introduction of advanced Real-World Evidence platforms like Komodo Health's MapEnhance, significantly impacting healthcare strategy formulation and drug development.
The Role of Geriatric Demographics and Technological Advancements
An aging population is one of the primary catalysts of growth. The escalating need for tailored clinical trial initiatives to address age-associated conditions is emphasized, considering the projected demographic trends towards a global surge in the geriatric populace. In parallel, the integration of cutting-edge technologies, from predictive analytics and AI to blockchain, is actively reshaping the market's dynamics. Major market contenders are leveraging these advancements to enhance the scope and efficacy of clinical trials.
Regional Dynamics and Market Services
While North America maintains its stronghold as the most considerable contributor to the market revenue, Asia-Pacific regions exhibit the fastest growth trajectory, as highlighted by the report's focused geographical analysis. An exploration into the array of services underlines the vital components that sustain the clinical trial supplies market. Among these services, logistics, supply chain management, and regulatory compliance are prominent, serving the expansive needs of pharmaceutical firms, CROs, and medical device companies.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 175 |
| Forecast Period | 2024-2028 |
| Estimated Market Value (USD) in 2024 | $2.75 Billion |
| Forecasted Market Value (USD) by 2028 | $3.72 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
A selection of companies mentioned in this report includes:
- Thermo Fisher Scientific Inc.
- IQVIA
- Eurofins Scientific SE
- Parexel International Corporation
- ICON PLC
- Catalent Inc.
- Intertek Group PLC
- Recipharm AB
- World Courier
- Almac Group Ltd.
- Piramal Pharma Solutions
- Clinigen Group PLC
- Movianto GmbH
- Marken Limited
- PCI Pharma Services
- Rubicon Research Private Limited
- Bionical Ltd.
- Durbin PLC
- SIRO Clinpharm Pvt. Ltd.
- Biocair International Ltd.
- Ancillare LP
- Myonex
- Klifo A/S
- Alium Medical Limited
- ADAllen Pharma
- Sharp Services LLC
For more information about this report visit https://www.researchandmarkets.com/r/m4le7r
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
