New York, May 08, 2024 (GLOBE NEWSWIRE) -- Overview
The Global In Vitro Diagnostics (IVD) market was valued at USD 106.9 Billion in 2023 which is further expected to reach USD 202.9 Billion in 2032 at a CAGR of 25.2%.
In vitro diagnostics (IVDs) are scientific tests designed to check upon diseases, conditions, and infections. The term "in vitro" refers to tests performed in controlled laboratory settings using test tubes and similar equipment. This distinguishes them from "in vivo" tests conducted inside the body. IVDs play a crucial role in diagnosing and monitoring various health issues, providing a non-invasive and accurate method to assess an individual's health.
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/in-vitro-diagnostics-ivd-market/request-sample

Important Insights
- Projections suggest a substantial surge, within the in vitro diagnostics (IVD) market as it is expected to show noteworthy growth by reaching USD 202.9 billion by 2032. This represents a significant Compound Annual Growth Rate (CAGR) of 7.4%, commencing from its 2023 base value of USD 106.9 billion.
- Reagent products lead the global in vitro diagnostics (IVD) market, securing the largest revenue share. This dominance is expected to persist due to significant R&D efforts by major industry players, introducing innovative reagents and specialized kits for rapid cancer detection.
- The molecular diagnostics category holds the major market share, driven by the introduction of groundbreaking products and continuous technological advancements. Notably, innovations like plasmofluidic chips, with their compact size, have revolutionized molecular diagnostics, emerging as the market leader.
- The infectious disease application dominates the in vitro diagnostics market, focusing on life-threatening infections. Collaborative partnerships among key players aim to enhance their cutting-edge laboratory services, strengthening the healthcare ecosystem by providing improved infectious disease diagnosis and management.
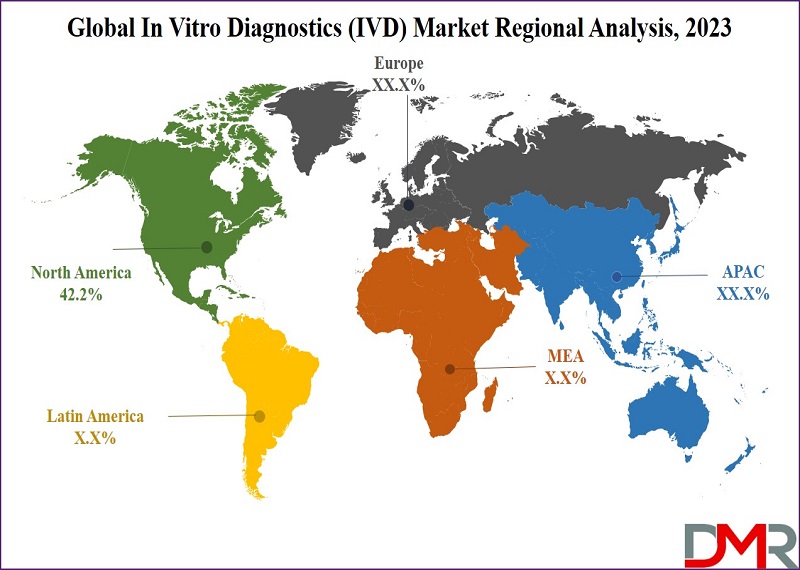
- North America, contributing 42.2% of 2023 in vitro diagnostics (IVD) revenue, maintains leadership, driven by factors like chronic diseases, an aging population, substantial healthcare funding, and rising demand for genetic testing.
- Asia Pacific emerges as a key in vitro diagnostics (IVD) growth region in 2023, propelled by stable economies, supportive healthcare policies, a growing middle class, and rapid urbanization.
Latest Trends
- Point-of-Care (POC) Diagnostics: Increased adoption of POC diagnostics amplifies market growth, offering accessible and rapid diagnostic solutions.
- Advanced Technology Integration: Integration of advanced technologies into IVD products enhances diagnostic capabilities, fostering market expansion.
- Personalized Medicine Awareness: Growing awareness and acceptance of personalized medicine and companion diagnostics contribute significantly to the market's growth trajectory.
- Genetic-Level Diagnostic Shift: The market experiences a transition from traditional to genetic-level diagnostics, leveraging advanced technologies like molecular diagnostics, genetic testing, PCR, and NGS.
In Vitro Diagnostics (IVD) Market: Competitive Landscape
- The competitive landscape of the global In vitro diagnostics market, led by key players like Siemens Healthcare, Abbott, Sysmex Corp, bioMerieux, and Qiagen, offers diverse features over a wide range of sectors. The In Vitro Diagnostic market is diverse and competitive, with global and regional players seeking market share. The absence of a dominant player results in varied market structures influenced by different-sized competitors.
- In March 2023, the FDA approval of Abbott's Alinity laboratory device for diagnosing traumatic brain injuries marks a significant advancement, providing healthcare professionals with a valuable tool for improving patient care in critical areas.
Some of the Prominent Market Players
- Siemens Healthcare
- Abbott
- Sysmex Corp
- bioMerieux
- Qiagen
- Danaher Corp
- Quest Diagnostics
- Becton Dickinson (BD)
- Quidel Corp
- Grifols
- Other Key Players
Transform your business approach with strategic insights from our report. Get in touch to request our brochure today! : https://dimensionmarketresearch.com/report/in-vitro-diagnostics-ivd-market/download-reports-excerpt
In Vitro Diagnostics (IVD) Market Scope
| Report Highlights | Details |
| Market Size (2023) | USD 106.9 Bn |
| Forecast Value (2032) | USD 202.9 Bn |
| CAGR (2024-2032) | 7.4% |
| Leading Region in terms of Revenue Share | North America |
| Percentage of Revenue Share by Leading Region | 42.2% |
| Historical Data | 2017 - 2022 |
| Forecast Data | 2025 – 2032 |
| Base Year | 2023 |
| Estimate Year | 2024 |
| Segments Covered | By Component, By Technology, By Application |
| Regional Coverage | North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
Market Analysis
In terms of product category, reagent products have the highest revenue share in 2023 as they dominate the global IVD market. This segment is projected to maintain its prominence, given high R&D efforts by such industry giants as driving the development of novel reagents and specialized kits for prompt cancer detection. The focus on advanced reagents and precise diagnostics highlights the importance of this segment for next-generation IVD, which could revolutionize medicine by identifying diseases quickly and more accurately.
Molecular diagnostics holds the largest market share, in terms of technology mainly due to new product introductions and technological improvements. Groundbreaking innovations, such as the compact plasmofluidic chips used for testing solutions highlight how molecular diagnostics play an ever-changing role in diagnosis technologies and their associated effects on healthcare supplies and management of diseases.
Growth Drivers
- High Disease Prevalence: The global in vitro diagnostics (IVD) market is propelled by the elevated occurrence of chronic and infectious diseases, driving demand for diagnostic procedures.
- Essential Healthcare Role: The IVD market continues to evolve, driven by its crucial role in diagnosing and managing a diverse range of health conditions, ensuring its sustained significance in healthcare.
Restraints
- Regulatory Challenges: Despite challenges such as stringent regulations and complex reimbursement procedures, the IVD market perseveres, evolving and expanding to meet healthcare demands.
- Reimbursement Issues: Inadequate compensation policies or delays in compensation decisions can discourage healthcare providers from adopting new IVD technology or tests.
- High Development Costs: Research and development costs for new diagnostic tests and technology may be substantial, especially considering the need for clinical validation and regulatory approval, which may also deter investment.
Growth Opportunities
- Technological Advancements: Continuous innovation in diagnostic technology, which includes molecular diagnostics, next-generation sequencing, and point-of-care testing, opens new avenues for IVD companies to develop advanced and more accurate diagnostic tests.
- Rising Disease Burden: The increasing occurrence of chronic and infectious disease globally, coupled with aging populations, creates a growing demand for diagnostic tests for early detection.
- Personalized Medicine: The shift toward personalized medicine and targeted treatment therapies necessitates the development of companion diagnostics to identify patient-specific biomarkers and guide treatment decisions, driving the demand for specialized IVD tests.
Purchase the Competition Analysis Dashboard Today: https://dimensionmarketresearch.com/checkout/in-vitro-diagnostics-ivd-market

In Vitro Diagnostics (IVD) Market Segmentation
By Product
- Instruments
- Reagents
- Services
By Technology
- Molecular Diagnostics
- Immunoassay
- Microbiology
- Hematology
- Clinical Chemistry
- Coagulation
- Others
By Application
- Infectious Disease
- Diabetes
- Oncology
- Nephrology
- Cardiology
- Drug Testing
- Autoimmune Disease
- Others
By End User
- Laboratories
- Hospitals
- Home Care
- Others
By Test Location
- Point of Care
- Home Care
- Others
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/in-vitro-diagnostics-ivd-market/request-sample
Regional Analysis
- North America holds a leading position in the global in vitro diagnostics (IVD) market, contributing 42.2% of total revenue. This dominance is sustained by factors such as a growing elderly population, increased healthcare funding, government support, and a rising demand for personalized healthcare through genetic testing, especially for conditions like diabetes and cancer.
- Following Asia Pacific holds a notable position in the 2023 in vitro diagnostics market, expected to be the fastest-growing region. Factors include strong economies, supportive healthcare policies, a growing middle class, and rapid urbanization. In this region, in vitro diagnostics tests are essential for diagnosing various health concerns, particularly heart-related disorders, infectious diseases, and cancer, contributing to their significance in industry growth.
By Region and Countries
North America
- The U.S.
- Canada
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Discover additional reports tailored to your industry needs
- In-Vitro Toxicology Market is expected to reach a value of USD 36.4 billion in 2023, and it is further anticipated to reach a market value of USD 104.8 billion by 2032 at a CAGR of 12.5%.
- ECG Patch and Holter Monitor Market size is estimated to have a value of USD 1.8 billion in 2024 and is expected to reach USD 9.6 billion by the end of 203 at a CAGR of 20.3% over the forecasted period.
- Insulin Delivery Devices Market is expected to dominate with USD 35.2 billion in 2024 and is anticipated to grow to USD 75.4 billion by 2033 at a CAGR of 8.8%
- Nutraceuticals Market size is anticipated to be valued at USD 367.3 billion in 2024 and is further predicted to reach USD 863.7 billion by 2033, at a CAGR of 10.0 %.
- Wheat Protein Market size is anticipated to be valued at USD 7.3 billion in 2024 and is further predicted to reach USD 10.7 billion by 2033, at a CAGR of 4.4 %.
- Pharmacogenomics Market is estimated to reach a market size of USD 6.9 billion which is foreseen to be USD 12.0 billion in 2033 with a CAGR of 6.3%.
- Acellular Dermal Matrices will reach a market value of 3.7 USD billion in 2024 and will surge higher to USD 9.8 billion by 2033 at a CAGR of 11.4% in the forecast period (2024 - 2033).
- Central Venous Catheter Market is projected to reach a market value of USD 1,220.4 million in 2024 and is further expected to attain a market value of USD 2,140.1 million by 2033 at a CAGR of 6.4%.
- Sleep Apnea Devices Market will reach the figure of USD 4.8 Billion in 2024 while it is further anticipated that the market will reach the value of USD 8.9 Billion of 2033 at a CAGR of 7.1%.
- Remote Health Monitoring Market is estimated to have a value of USD 7.8 billion in 2024 and is expected to rise to USD 38.1 billion by the end of 2033
Recent Developments in the In Vitro Diagnostics (IVD) Market
- March 2023: NeoGenomics expands its oncology testing offerings, including Neo Comprehensive - Solid Tumor and Neo Comprehensive - Myeloid Disorders genomic profiles for cancer diagnosis.
- February 2023: Invitae introduces CE-IVD Cancer Testing Kits in Europe, featuring FusionPlex Dx and LiquidPlex Dx, marking the launch of its diagnostic tools.
- November 2022: Bio Rad Laboratories Inc. formed a collaboration with Abbott Laboratories for their catalog expansion by introducing quality control products.
- October 2022: UK-based Binding Site Group was acquired by Thermo Fisher Scientific Inc. to expand their specialty diagnostic sector.
- February 2023: Thermo Fisher introduced Renvo Rapid PCR Test for in-air SARS-CoV-2 detection using AerosolSense Sampler, boosting the growth of the in-vitro diagnostics market.
- January 2023: FUJIFILM Sonosite launched the Sonosite PX ultrasound system in India, aiming to enhance clinician ergonomics and efficiency.
- July 2022: BioGX launches CE-marked POC three-gene multiplex COVID-19 test on the Pixl platform.
About Dimension Market Research (DMR):
Dimension Market Research (DMR) is a market research and consulting firm based in India & US, with its headquarters located in the USA (New York). The company believes in providing the best and most valuable data to its customers using the best resources analysts work, to create unmatchable insights into the industries, and markets while offering in-depth results of over 30 industries, and all major regions across the world.
We also believe that our clients don’t always want what they see, so we provide customized reports as well, as per their specific requirements to create the best possible outcomes for them and enhance their business through our data and insights in every possible way.
