LAS VEGAS, Nov. 1, 2006 (PRIMEZONE) -- Samaritan Pharmaceuticals Inc. (AMEX:LIV), a developer of innovative drugs, announced today it has completed patient enrollment of its Phase IIb (Stage 2) 28 day monotherapy clinical trial, testing Samaritan's lead "orally-available" entry inhibitor HIV drug SP-01A, to treat HIV drug resistance.
A photo accompanying this release is available at http://www.primezone.com/newsroom/prs/?pkgid=2993
Dr. Janet Greeson, CEO of Samaritan Pharma stated, "We are very optimistic about the future of SP-01A as a drug to rescue patients experiencing frightful HIV drug resistance."
SP-01A MECHANISM OF ACTION
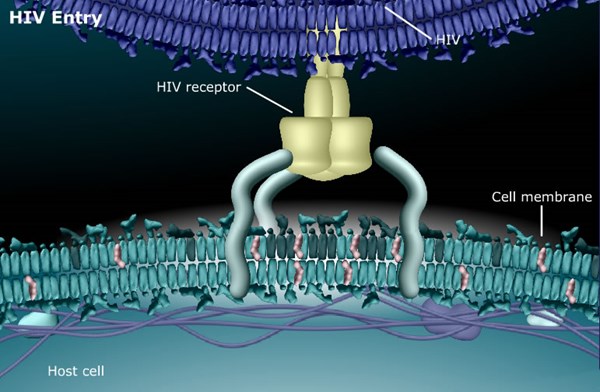
SP-01A appears to block HIV entry into healthy cells by reducing intracellular cholesterol, subsequently interfering with HIV's mechanism of entry as HIV needs cholesterol in a cell's membrane to enter healthy cells. Since SP-01A targets the cell membrane rather than the HIV virus itself, it is believed to have decided advantages since it points toward SP-01A being less susceptible to developing "drug resistance," a common occurrence with most HIV drugs on the market today.
http://www.samaritanpharma.com/html/hivtrials.html
For more information on this clinical trial, please go to the FDA Clinical Trials Website at www.clinicaltrials.gov.
Samaritan Pharmaceuticals: "We LIV....to Save Lives."
Samaritan is a small-cap Biotech, driven to discover, develop and commercialize innovative therapeutics for AIDS, Alzheimer's, Cancer and Heart disease patients. Look at www.samaritanpharma.com. Please register on Website so we can notify you of upcoming conference calls, news and events.
The Samaritan Pharmaceuticals Inc. logo is available at http://www.primezone.com/newsroom/prs/?pkgid=2670
Disclaimer
The company disclaims any information that is created by an outside party and endorses only information that is communicated by its press releases, filings and Website. This news release contains forward-looking statements that reflect management's current beliefs about the potential for its drug candidates, science and technology. However, as with any biopharmaceutical under development, there are significant risks and uncertainties in the process of development and regulatory review. There are no guarantees that products will prove to be commercially successful. For additional information about the factors that affect the company's business, please read the company's latest Form 10-K filed April 13, 2006. The company undertakes no duty to update forward-looking statements.
The photo is also available at NewsCom, (www.newscom.com), and via AP PhotoExpress.