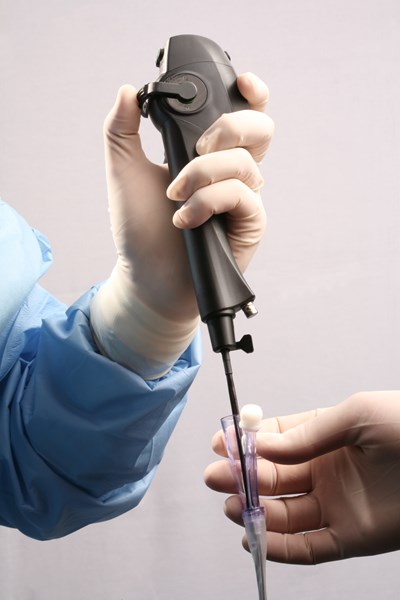
ORANGEBURG, N.Y., Sept. 12, 2007 (PRIME NEWSWIRE) -- Vision-Sciences, Inc. (Nasdaq:VSCI) ("Vision-Sciences" or the "Company") announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration ("FDA") to market the Company's new line of advanced digital, video-based flexible endoscopes, which come with an integrated "built-in" light source, eliminating the need for a separate camera head, light cable and optical coupler.
Photos accompanying this release are available at
http://www.primenewswire.com/newsroom/prs/?pkgid=4181
http://www.primenewswire.com/newsroom/prs/?pkgid=4182
The ENT (Ear, Nose and Throat) and TNE (Transnasal Esophagoscopy) scopes are the first two in the series of uniquely advanced digital endoscopy platforms to be introduced by Vision-Sciences. These lightweight videoscopes facilitate diagnostic and therapeutic procedures with the introduction of the world's smallest diameter insertion tube that contains a high resolution, tiny CCD (charge coupled device) camera at the tip of the insertion tube, offering a sharp, high definition, vibrant full screen image.
Moreover, these advanced videoscopes are the first in the world that do not contain difficult-to-clean operating channels associated with scopes from other manufacturers. Instead, Vision-Sciences scopes are used in conjunction with the Company's patented, disposable, sterile Slide-On(r) EndoSheath(r) technology that covers the entire scope. The proprietary EndoSheath(r) Technology not only isolates the endoscope from the patient, but contains a disposable operative channel, which eliminates instrumentation and patient tissue biopsies from coming in contact with the reusable scope, unlike when passed through the built-in channel of conventional scopes from other manufacturers. The EndoSheath(r) Technology eliminates the need for elaborate high-level disinfection between procedures, providing rapid equipment turnover, limiting capital investment for additional scope inventory, reducing exposure to toxic chemicals, and dramatically lowering repair and maintenance costs.
The ENT-5000 flexible video laryngoscope is inserted in the nose down to the throat, providing precise, vivid images of the internal structures of the nasal cavity, vocal folds, larynx and other areas of the throat. The TNE-5000 flexible video transnasal esophagoscope allows equally excellent visualization further down the esophagus and all the way down to the stomach. These advanced videoscopes will be used to examine patients suffering from various disorders, ranging from difficulty in swallowing (Dysphagia), difficulty in breathing, sleep apnea, asthma, chronic cough, gastroesophageal reflux disease (GERD), Barrett's disease and symptoms of cancer.
"We believe this is a breakthrough in endoscopy," stated CEO, Mr. Ron Hadani. "Our new videoscope with the EndoSheath(r) technology is poised to ultimately replace prevalent old fiber-optic technology. While Gastroenterologists have been using costly conventional video endoscopes for years, other specialties were forced to use the old 'eye to the eye-piece' fiber technology due to high conversion costs. This is the first time physicians will have the opportunity to have the world's best videoscope uniquely combined with the practice-efficiency of the EndoSheath(r) Technology. We are planning to be aggressive in pricing in order to commence a revolution and drive rapid adoption of video technology throughout the medical community."
The Company has also successfully completed viral microorgranism "barrier testing" per FDA guidance for the videoscope EndoSheath(r) Technology, proving that the EndoSheath(r) barrier is effective to microorganisms as small as 27 nanometers -- the FDA's benchmark for barriers. All other dangerous microorganisms such as the AIDS (80-120 nanometers), Hepatitis-C (30-60 nanometers) or Herpes Simplex Virus (150-200 nanometers) are significantly larger. Vision-Sciences has successfully performed barrier testing on the complete line of EndoSheath(r) products and remains focused on delivering the best in endoscopy infection control, specifically designed for a demanding health care environment.
"With increasing global vigilance on the prevention of hospital acquired infections, we believe our EndoSheath(r) technology offers unique and relevant advantages to multitudes of patients and healthcare practitioners," stated Mr. Carlos Babini, Executive Vice President of Vision-Sciences. "We test this innovative EndoSheath barrier with nano-level scrutiny and remain committed to continued research on the clinical efficacy of EndoSheath(r) Technology, such as the recent study concluded by Dr. Alvarado at the University of Wisconsin."
The ENT Slide-On(r) EndoSheath(r) System was recently featured in an award winning study led by the world renowned Carla Alvarado, PhD, at the APIC annual meeting in May this year. Dr. Alvarado evaluated the clinical efficacy of the EndoSheath(r) barrier and potential bacterial contamination following use. The study found that not only did the EndoSheath(r) barrier remain intact, but after the recommended handling procedures, no bacteria could be cultured from the endoscope.
"We leave incremental endoscope improvements to others," stated Mr. Hadani. "At Vision-Sciences we are transforming video-based endoscopy with meaningful integration of enabling components, to provide extraordinary vision and superior endoscopic systems, facilitating quality healthcare."
About Vision-Sciences:
Vision-Sciences, Inc. (Nasdaq:VSCI) develops, manufactures and markets unique flexible endoscopic products for the urology, gastroenterology and the ENT markets, utilizing the proprietary sterile disposable EndoSheath(r) Technology. This technology provides quick and efficient equipment turnover, and ensures the patient a contaminant-free endoscope insertion.
The Vision-Sciences, Inc. logo is available at http://www.primenewswire.com/newsroom/prs/?pkgid=3876
The photos are also available via AP PhotoExpress.