BEVERLY, MA--(Marketwire - Mar 11, 2013) - Cellceutix Corporation (
Retinoblastoma is a rare and serious disease in which cancer cells grow in the retina of the eye. According to the American Cancer Society, less than 300 children are diagnosed with retinoblastoma each year in the United States. It is the most common type of eye cancer in children and accounts for 6 percent of all cancers in children under the age of five. It is most frequently diagnosed in infants and very young children; the average age of children when they are diagnosed is 2. (www.cancer.org/cancer/retinoblastoma)
Current therapies are difficult to tolerate especially in children, e.g., removal of the eye, intense radiation therapy, or chemotherapy given either systemically or locally in the eye, and are limited in their effectiveness. The need for an effective therapy against retinoblastoma is urgently needed.
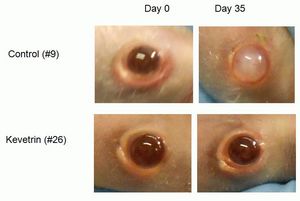
The Company conducted pre-clinical studies using human retinoblastoma cells (WERI-Rb-1) in nude mice that were implanted either subcutaneously or directly into the eye, intravitreally. Treatment with Kevetrin significantly reduced the tumor volume by more than half in the subcutaneous tumor model and showed a significant improvement in the clarity of the eye in mice treated with Kevetrin as shown in the photographs below and on our website at www.cellceutix.com/kevetrin.
Given the limited treatment options, devastating effects, and small patient population, Cellceutix believes that retinoblastoma would make an excellent candidate for a phase 2/3 trial once our present phase 1 trial is completed. It would qualify for a number of FDA programs (Orphan, Fast Track and Breakthrough) that can exponentially shrink development time, should clinical data support the current research.
"While our Phase 1 clinical trial is ongoing, we are planning for future trials that are aimed directly at the latest initiatives of the Food and Drug Administration to expedite development of 'breakthrough' technologies to commercialization," commented Dr. Krishna Menon, Chief Scientific Officer at Cellceutix. "The data from the studies affirm that Kevetrin may be useful in a new tumor type, in addition to the other indications that we are studying. Kevetrin has delivered robust results against all indications tested to date. Cellceutix's strategy is to have multiple trials ongoing against multiple cancer types concurrently. We are conducting testing against several more cancers to delineate the quickest path to market to build corporate and shareholder value."
About Kevetrin™
As a completely new class of chemistry in medicine, Kevetrin™ has significant potential to be a major breakthrough in the treatment of solid tumors. Mechanism of action studies showed Kevetrin's unique ability to affect both wild and mutant types of p53 (often referred to as the "Guardian Angel Gene" or the "Guardian Angel of the Human Genome") and that Kevetrin strongly induced apoptosis (cell death), characterized by activation of Caspase 3 and cleavage of PARP. Activation of p53 also induced apoptosis by inducing the expression of p53 target gene PUMA. p53 is an important tumor suppressor that acts to restrict proliferation by inducing cell cycle checkpoints, apoptosis, or cellular senescence.
In more than 50 percent of all human carcinomas, p53 is limited in its anti-tumor activities by mutations in the protein itself. Currently, there are greater than 10 million people with tumors that contain inactivated p53, while a similar number have tumors in which the p53 pathway is partially abrogated by inactivation of other signaling components. This has left cancer researchers with the grand challenge of searching for therapies that could restore the protein's protective function, which Kevetrin appears to be doing the majority of the time.
Further information on the clinical trial, titled "A Phase 1, Open-Label, Dose-Escalation, Safety, Pharmacokinetic and Pharmacodynamic Study of Kevetrin (Thioureidobutyronitrile) Administered Intravenously, in Patients With Advanced Solid Tumors," is available at: http://clinicaltrials.gov/ct2/show/NCT01664000?term=cellceutix&rank=1
About Cellceutix
Headquartered in Beverly, Massachusetts, Cellceutix is a publicly traded company under the symbol "CTIX". It is an emerging bio-pharmaceutical company focused on the development of its pipeline of compounds targeting areas of unmet medical need. Our flagship compound, Kevetrin™, is an anti-cancer drug which has demonstrated the ability in pre-clinical studies to regulate the p53 pathway and attack cancers which have proven resistant to today's cancer therapies (drug-resistant cancers). Cellceutix also owns the rights to seven other drug compounds, including KM-133, which is in development for psoriasis, and KM-391 for the treatment of the core symptoms of autism. More information is available on the Cellceutix web site at www.cellceutix.com.
Safe Harbor Forward-Looking Statements
To the extent that statements in this press release are not strictly historical, including statements as to revenue projections, business strategy, outlook, objectives, future milestones, plans, intentions, goals, future financial conditions, future collaboration agreements, the success of the Company's development, events conditioned on stockholder or other approval, or otherwise as to future events, such statements are forward-looking, and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements contained in this release are subject to certain risks and uncertainties that could cause actual results to differ materially from the statements made. Factors that may impact Cellceutix's success are more fully disclosed in Cellceutix's most recent public filings with the U.S. Securities and Exchange Commission.
Contact Information:
Cellceutix Corp.
Leo Ehrlich
(978) 236-8717