BEDMINSTER, N.J., and DUBLIN, Ireland, May 9, 2013 (GLOBE NEWSWIRE) -- Amarin Corporation plc (Nasdaq:AMRN), a late-stage biopharmaceutical company focused on the commercialization and development of therapeutics to improve cardiovascular health, today announced financial results for the quarter ended March 31, 2013 and provided an update on company operations.
Key Amarin accomplishments since the quarter ended December 31, 2012 include:
- Launched Vascepa® (icosapent ethyl) capsules in the United States on January 28, 2013 for the MARINE indication (use as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia)
- Recognized $2.34 million in product revenue from Vascepa sales in Q1 in accordance with GAAP ($5.2 million in net value of Vascepa was sold to wholesalers in Q1, resulting in $2.9 million of deferred product revenue under GAAP in Q1)
- Secured formulary access for Vascepa with over 190 million lives now covered by payors without restrictions, including 40 million converted to Tier 2 in April and May
- Received Food and Drug Administration (FDA) acceptance for review of supplemental New Drug Application (sNDA) seeking approval for the marketing and sale of Vascepa for the ANCHOR indication (use as an adjunct to diet in the treatment of adult patients with high triglycerides (TG ≥200 mg/dL and <500 mg/dL) with mixed dyslipidemia)
- Reported statistically significant reductions of apolipoprotein C-III (Apo C-III) of 25.1% and 19.2%, compared to placebo, as demonstrated by Vascepa in post-hoc analyses of the MARINE and ANCHOR Phase 3 clinical trials, respectively
- Received FDA approval of two additional active pharmaceutical ingredient (API) suppliers, BASF and Chemport, for the manufacture of Vascepa giving Amarin three qualified API suppliers
- Increased patents issued or allowed in the United States to 22 (adding 11 in the first quarter alone, including 3 since our last patent announcement), all but two of which have patent terms extending into 2030, with more than 30 additional patent applications being prosecuted in the United States alone
"On January 28, 2013 Amarin launched Vascepa for use in its initial indication as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia, the MARINE indication," said Joseph Zakrzewski, Chairman and Chief Executive Officer of Amarin. "Since that day, we have seen meaningful progress in multiple areas of our commercialization strategy, including uptake in Vascepa utilization by physicians in both the specialty and primary care communities, the initial migration of managed care lives from Tier 3 to Tier 2 coverage, the continued expansion of our patent portfolio, the further strengthening of our supply chain, and the acceptance for review by the FDA of our sNDA for the ANCHOR indication, which, if approved, would enable promotion of Vascepa to a significantly larger patient population."
Operational update
Commercialization update
Amarin's direct sales force, consisting of approximately 275 sales professionals, made sales calls to clinicians for two months in Q1 2013. Amarin reports that, since launch, access to clinicians has been good, and that it has yet to hear any significant negative reaction to the efficacy or safety profile of Vascepa. Amarin's sales professionals are currently targeting the limited group of clinicians who are the highest prescribers of other lipid therapies. Vascepa is being marketed for use as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia, the initial indication for Vascepa. Amarin believes that Vascepa is well differentiated in this market based on its safety profile, which is similar to placebo, and its spectrum of demonstrated lipid benefit at 4g/day, including statistically significant reductions in triglycerides, Apo B, VLDL-C, and non-HDL-C, with no increase in LDL-C, also known as bad cholesterol.
A chart accompanying this press release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=18620
Since launching Vascepa, Amarin has:
- Witnessed steady increases in the number of clinicians prescribing Vascepa (now over 4,000), in the number of prescriptions reported (via third-party sources), in the number of bottles of Vascepa shipped from wholesalers to retail pharmacies and in the number of co-pay cards used by patients
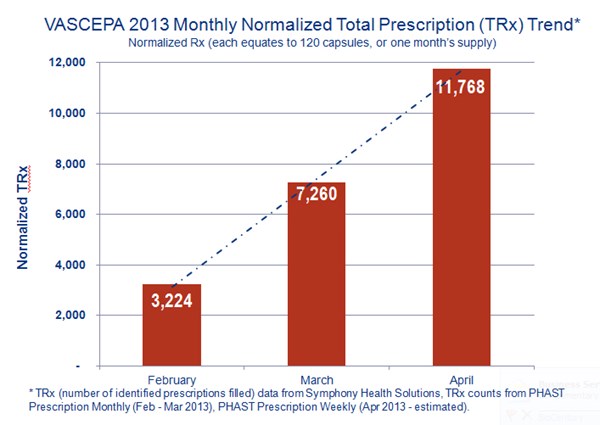
- Witnessed monthly volume (via third-party sources, often underestimated) increase with consistency in the first months post launch with prescriptions increasing from 3,224 to 7,260 to 11,768 normalized TRx, reported in February, March, and April, respectively (normalized TRx equates to 120 capsules, or one month's supply)
- Focused on clinician education about Vascepa's clinical results and increased product awareness
- Secured early managed care coverage based on the safety and efficacy profile of Vascepa, including initial Tier 2 conversions (without restrictions) in April and May, noting that it typically takes two-to-three months of time to translate into script data
- Trained clinicians as speakers on behalf of Vascepa and conducted speaker programs for groups of healthcare professionals
- Mitigated the Tier 3 vs. Tier 2 co-payment differential for patients with an actively used co-pay discount program (a common program for a new drug until Tier 2 coverage is secured)
- Received early, but encouraging, feedback from clinicians regarding their patients' initial experiences with Vascepa
Vascepa additional indication progress
In parallel with marketing Vascepa for the MARINE indication, Amarin is pursuing approval of Vascepa for the considerably larger ANCHOR indication. In a clinical trial of the use of Vascepa in the ANCHOR indication, as previously announced, Vascepa demonstrated statistically significant reductions in a broad spectrum of lipid and inflammatory markers, on top of optimized statin therapy, including significant reduction in LDL-C. In April 2013, as previously announced, the FDA accepted for review Amarin's sNDA for the ANCHOR indication that, upon approval, would enable Amarin to market and sell Vascepa for use in the ANCHOR indication. The FDA assigned a PDUFA action date of December 20, 2013 for this sNDA, which date is consistent with the standard FDA ten-month review period. The safety results from the ANCHOR trial are included in the current label for Vascepa. At a daily Vascepa dose of 4 grams, all of the primary and secondary efficacy endpoints of the ANCHOR trial were achieved. As a result, Amarin is optimistic that the FDA will approve Vascepa for this indication.
Vascepa supply update
In the fourth quarter of 2012, Amarin submitted two sNDAs, one each for two additional active pharmaceutical ingredient (API) suppliers for Vascepa: BASF and Chemport. Both of these sNDA filings were approved in April 2013. Qualification of these suppliers is part of Amarin's strategy to expand its supply chain to provide greater capacity to meet anticipated demand, enable supply diversification and flexibility and introduce cost competition among high quality suppliers. With the approval of these suppliers, Amarin now has three qualified API suppliers for Vascepa, enabling Amarin to potentially reduce supply costs by 50% or more.
Vascepa exclusivity update
Amarin continues to make significant progress in its effort to expand patent protection for Vascepa and now has 22 patents issued or allowed in the United States with over 30 additional U.S. patent applications being prosecuted. This patent portfolio includes claims covering Vascepa's pharmaceutical composition and methods of use for the MARINE indication, ANCHOR indication and other potential uses of Vascepa. Amarin is also pursuing patent applications related to Vascepa in multiple jurisdictions outside the United States. All but two of the granted patents have expiry dates extending into 2030 and the majority of patent applications, if and when allowed, are anticipated to have expiry dates in or beyond 2030. Patent protection for Vascepa is augmented by protection provided by trade secrets, manufacturing barriers to entry and three- or five-year regulatory exclusivity.
REDUCE-IT and other Vascepa-related clinical development
Amarin continues to progress patient enrollment in its REDUCE-IT cardiovascular outcomes study with more than 4,000 patients enrolled in the study to date. Amarin anticipates continuing to enroll patients in this study throughout 2013. Results of the study will not be available until a specified number of cardiovascular events have been observed, the timing of which is not expected in the near-term.
Financial update
Amarin reported cash and cash equivalents of $201.8 million at March 31, 2013.
Amarin reported net product revenues for the quarter ended March 31, 2013 of $2.34 million under U.S. Generally Accepted Accounting Principles (GAAP). In accordance with GAAP, until Amarin has more experience in the commercialization of Vascepa, Amarin plans to recognize revenue based on the resale of Vascepa by the wholesalers to which Amarin sells Vascepa, and not based on sales from Amarin to such wholesalers. During the quarter ended March 31, 2013, the net value of Vascepa sold to wholesalers was $5.2 million, which, in accordance with GAAP, resulted in $2.9 million of deferred product revenue from Vascepa sales in the quarter ended March 31, 2013.
Consistent with industry practice, the net price of Vascepa in the quarter ended March 31, 2013 reflects the deduction of one-time discounts paid to wholesalers to stock Vascepa in advance of the launch of Vascepa on January 28, 2013 as well as the costs of Amarin's 2013 co-payment rebate card program and customary payor rebates and allowances.
Cost of goods sold during the quarter ended March 31, 2013 was $1.3 million. All of the API sold during the first quarter of 2013 was sourced from a single API supplier. As previously commented, Amarin's purchases of API from that supplier in 2012 and Q1 2013 are at a higher cost/kg than scheduled future purchases from such supplier. The unusually high cost of goods percentage is attributable to start up costs, geography, special launch related discounts to wholesalers, exchange rate exposure, lower volume, our co-payment rebate card program and less favorable terms than exist with other suppliers. Amarin expects steady state gross margins to approach the high seventies to eighty percent level. In future periods, Amarin also anticipates purchasing API from BASF and Chemport, the sNDAs for which were approved in April 2013. The API cost/kg from BASF and Chemport are also significantly lower than the costs incurred for past purchases of API from our single initial supplier.
Under GAAP, Amarin reported a net loss of $62.2 million in the first quarter of 2013, or basic and diluted loss per share of $0.41. This net loss included $4.9 million in non-cash share-based compensation expense, $0.5 million in non-cash warrant compensation income, and a $3.6 million gain on the change in the fair value of derivatives. In the first quarter of 2012, GAAP net loss was $88.3 million, or basic and diluted loss per share of $0.65, and included $3.9 million in non-cash share-based compensation expense, $2.4 million in non-cash warrant compensation expense, and a $66.2 million loss on the change in the fair value of derivatives.
Excluding non-cash gains or losses for share-based compensation, warrant compensation and change in value of derivatives, non-GAAP adjusted net loss was $61.4 million for the first quarter of 2013, or non-GAAP adjusted basic and diluted loss per share of $0.41, compared to non-GAAP adjusted net loss of $15.8 million, or non-GAAP adjusted basic and diluted loss per share of $0.12 for the same period in 2012.
During the three months ended March 31, 2013, net cash decreased by approximately $58.4 million, including approximately $32.0 million paid for sales and marketing related expenses in connection with the initial commercial launch of Vascepa, approximately $13.0 million paid in support of the REDUCE-IT cardiovascular outcomes study and approximately $11.8 million for Vascepa API purchased in connection with the buildup of our commercial supply and for clinical trial material. During the three months ended March 31, 2013, research and development expense included $3.0 million for API from suppliers which were not approved until April 2013 which, for accounting purposes, was expensed in the period received.
As of March 31, 2013, Amarin had approximately 150.7 million ADSs outstanding as well as approximately 9.9 million, 11.3 million, and 0.9 million equivalent shares underlying warrants, stock options, and restricted or deferred stock units, respectively, at average exercise prices of $1.44, $7.45 and $8.49, respectively. In addition, our $150 million exchangeable senior notes issued in January 2012 are exchangeable prior to October 15, 2031 into an aggregate of 17.0 million ADSs (based on an initial exchange price of approximately $8.81 per ADS), subject to certain specified conditions. The notes accrue interest at an annual rate of 3.5%, payable semiannually in arrears on January 15 and July 15, beginning July 15, 2012. The notes will mature on January 15, 2032, unless earlier repurchased or redeemed by the company or exchanged by the holders.
Amarin's 2013 operational priorities
Operational priorities in 2013 are:
- Increasing revenues from sales of Vascepa
- Continuing managed care migration from Tier 3 to Tier 2 coverage
- Gaining approval of the ANCHOR indication sNDA (PDUFA date of December 20, 2013)
- Planning for the commercialization of the ANCHOR indication
- Obtaining additional patent awards from the USPTO
- Continuing development of a fixed-dose combination of Vascepa and a leading statin
- Submitting an sNDA for a fourth API supplier
- Publishing additional data from Amarin's clinical trials
- Obtaining FDA exclusivity determination
Conference call and webcast information
Amarin will host a conference call at 4:30 p.m. EDT (8:30 p.m. UTC/GMT) today, May 9, 2013. To participate in the call, please dial (877) 407-8033 within the United States or (201) 689-8033 from outside the United States. A replay of the call will be made available for a period of two weeks following the conference call. To hear a replay of the call, dial (877) 660-6853 (inside the U.S.) or (201) 612-7415 (outside the U.S.). A replay of the call will also be available through Amarin's website shortly after the call. For both dial-in numbers please use conference ID 411140. The conference call can also be heard live through the investor relations section of Amarin's website at www.amarincorp.com.
Use of non-GAAP adjusted financial information
Included in this press release and the conference call referenced above are non-GAAP adjusted financial information as defined by U.S. Securities and Exchange Commission Regulation G. The GAAP financial measure most directly comparable to each non-GAAP adjusted financial measure used or discussed, and a reconciliation of the differences between each non-GAAP adjusted financial measure and the comparable GAAP financial measure, are included in this press release after the condensed consolidated financial statements.
Non-GAAP adjusted net loss was derived by taking GAAP net loss and adjusting it with non-cash gains or losses for share-based compensation, warrant compensation, and change in value of derivative. Management believes that these non-GAAP adjusted measures provide investors with a better understanding of the company's historical results from its core business operations.
While management believes that these non-GAAP adjusted financial measures provide useful supplemental information to investors regarding the underlying performance of the company's business operations, investors are reminded to consider these non-GAAP measures in addition to, and not as a substitute for, financial performance measures prepared in accordance with GAAP. Non-GAAP measures have limitations in that they do not reflect all of the amounts associated with the company's results of operations as determined in accordance with GAAP. In addition, it should be noted that these non-GAAP financial measures may be different from non-GAAP measures used by other companies, and management may utilize other measures to illustrate performance in the future.
About Vascepa® (icosapent ethyl) capsules
Vascepa® (icosapent ethyl) capsules, known in scientific literature as AMR101, is a highly pure-EPA omega-3 prescription product in a 1 gram capsule.
Indications and Usage
- Vascepa (icosapent ethyl) is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia.
- The effect of Vascepa on the risk for pancreatitis and cardiovascular mortality and morbidity in patients with severe hypertriglyceridemia has not been determined.
Important Safety Information for Vascepa
- Vascepa is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to Vascepa or any of its components and should be used with caution in patients with known hypersensitivity to fish and/or shellfish.
- The most common reported adverse reaction (incidence >2% and greater than placebo) was arthralgia (2.3% for Vascepa, 1.0% for placebo).
FULL VASCEPA PRESCRIBING INFORMATION CAN BE FOUND AT WWW.VASCEPA.COM.
Forward-looking statements
This press release contains forward-looking statements, including statements about the commercial launch of Vascepa, including the number of total prescriptions to date and sales trends, expectations for revenue growth, product awareness, receptivity of clinicians to and patient experience with Vascepa; expectations regarding managed care migration from Tier 3 to Tier 2 coverage and continued growth in Tier 2 coverage; the pricing terms of commercial supply for Vascepa; expectations regarding gross margins and cost of goods sold (COGS); the timing of FDA decisions regarding Amarin's sNDA for the ANCHOR indication and regulatory exclusivity; the efficacy, safety and therapeutic benefits of Vascepa; the ability of Amarin to develop a fixed-dose combination of Vascepa and a leading statin; Amarin's ability to obtain sufficient patent protection and regulatory exclusivity for its product and product candidates, maintain trade secrets, and take advantage of manufacturing barriers to entry; continued enrollment of patients in Amarin's REDUCE-IT cardiovascular outcomes study; continued publication of study data; and continued assessment of collaboration prospects for commercialization of Vascepa. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. In particular, as disclosed in its previous filings with the U.S. Securities and Exchange Commission, Amarin's ability to effectively commercialize Vascepa will depend in part on its ability to create market demand for Vascepa through education, marketing and sales activities, to achieve market acceptance of Vascepa, to receive adequate levels of reimbursement from third-party payers, to develop and maintain a consistent source of commercial supply at a competitive price, and to obtain and maintain patent protection and regulatory exclusivity. Among the factors that could cause actual results to differ materially from those described or projected herein include the following: uncertainties associated generally with research and development, clinical trials and related regulatory approvals; the risk that Special Protocol Assessment agreements with the FDA are not a guarantee that FDA will approve a product candidate upon submission; the risk that the FDA may not complete its review of the ANCHOR sNDA by the PDUFA action date or grant new chemical entity regulatory exclusivity to Vascepa; the risk that historical REDUCE-IT clinical trial enrollment and randomization rates may not be predictive of future results and related cost may increase beyond expectations; the risk that patent applications may not result in issued patents, trade secrets may not be maintained and that circumstances that create manufacturing barriers to entry may not last; the risk that Amarin may not enter into a collaboration agreement for the commercialization of Vascepa in the ANCHOR indication under favorable terms or at all or market the ANCHOR indication successfully; and the risk that publications of scientific data may not accept proposals to publish Vascepa data. A further list and description of these risks, uncertainties and other risks associated with an investment in Amarin can be found in Amarin's filings with the U.S. Securities and Exchange Commission, including its most recent Quarterly Report on Form 10-Q. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Amarin undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Vascepa has been approved for use by the FDA as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Vascepa is under various stages of development for potential use in other indications that have not been approved by the FDA. Nothing in this press release should be construed as marketing the use of Vascepa in any indication that has not been approved by the FDA.
Important information regarding prescriptions data and product revenue
The historical prescription data provided in this press release is based on data published by a third party as of May 5, 2013. Although Amarin believes these data are prepared on a period to period basis in a manner that is generally consistent and that such results are indicative of current prescription trends, these data are based on estimates and should not be relied upon as definitive. These data may overstate or understate actual prescriptions. Based on other data available to Amarin and the history of such third-party prescription estimates in the early stages of launch of other new pharmaceutical products, Amarin believes that while the trends provided by this information are useful to gauge current prescription levels, such third-party methods have historically often understated actual prescriptions. Amarin commenced its commercial launch of Vascepa on January 28, 2013. Accordingly, there is a very limited amount of information available at this time to determine the actual number of total prescriptions for Vascepa. Amarin believes that investors should view these data with caution, as data for this single and limited period may not be representative of a trend consistent with the results presented or otherwise predictive of future results. Seasonal fluctuations in pharmaceutical sales, for example, may affect future prescription trends of Vascepa as could change in prescriber sentiment and other factors. Amarin believes investors should consider its results during this quarter together with its results over several future quarters, or longer, before making an assessment about potential future performance.
The photo is also available at Newscom, www.newscom.com, and via AP PhotoExpress.
| CONSOLIDATED BALANCE SHEET DATA | ||||
| (U.S. GAAP) | ||||
| Unaudited | ||||
| March 31, 2013 | December 31, 2012 | |||
| (in thousands) | ||||
| ASSETS | ||||
| Current Assets: | ||||
| Cash and cash equivalents | $ 201,780 | $ 260,242 | ||
| Restricted cash | 1,400 | ----- | ||
| Accounts receivable | 3,441 | ----- | ||
| Inventory | 27,435 | 21,262 | ||
| Deferred tax asset | 937 | 937 | ||
| Other current assets | 7,051 | 3,253 | ||
| Total Current Assets | $ 242,044 | $ 285,694 | ||
| Property, plant and equipment, net | 766 | 811 | ||
| Deferred tax asset | 11,993 | 8,044 | ||
| Other non-current assets | 5,335 | 4,951 | ||
| Intangible asset, net | 11,193 | 11,355 | ||
| Total Assets | $ 271,331 | $ 310,855 | ||
| LIABILITIES AND STOCKHOLDERS' EQUITY (DEFICIT) | ||||
| Current Liabilities: | ||||
| Accounts payable | $ 22,945 | $ 17,458 | ||
| Accrued interest payable | 4,646 | 2,520 | ||
| Deferred revenue | 2,865 | ----- | ||
| Accrued expenses and other liabilities | 11,935 | 5,224 | ||
| Total current liabilities | $ 42,391 | $ 25,202 | ||
| Long Term Liabilities: | ||||
| Warrant derivative liability | 49,011 | 54,854 | ||
| Exchangeable senior notes | 137,735 | 134,250 | ||
| Long term debt | 85,873 | 85,153 | ||
| Long term debt redemption feature | 15,600 | 14,577 | ||
| Other long term liabilities | 807 | 816 | ||
| Total liabilities | $ 331,417 | $ 314,852 | ||
| Stockholders' Deficit: | ||||
| Common stock | 124,846 | 124,597 | ||
| Additional paid-in capital | 625,086 | 619,266 | ||
| Treasury stock | (217) | (217) | ||
| Accumulated deficit | (809,801) | (747,643) | ||
| Total stockholders' deficit | $ (60,086) | $ (3,997) | ||
| Total Liabilities and Stockholders' Deficit | $ 271,331 | $ 310,855 | ||
| CONSOLIDATED STATEMENTS OF OPERATIONS DATA | ||
| (U.S. GAAP) | ||
| Unaudited | ||
| Three Months Ended March 31 | ||
| (in thousands, except share and per share amounts) | ||
| 2013 | 2012 | |
| Product Revenues | $ 2,341 | $ ----- |
| Operating Expenses: | ||
| Cost of goods sold | 1,287 | ----- |
| Marketing, general and administrative(1) | 39,267 | 14,027 |
| Research and development(1) | 21,838 | 4,756 |
| Total operating expenses | 62,392 | 18,783 |
| Operating loss | (60,051) | (18,783) |
| Gain (loss) on change in fair value of derivative liabilities(2) | 3,620 | (66,209) |
| Interest expense, net | (8,860) | (3,951) |
| Other (expense) income, net | (124) | 68 |
| Loss from operations before taxes | (65,415) | (88,875) |
| Benefit for income taxes | 3,257 | 590 |
| Net and comprehensive loss | $ (62,158) | $ (88,285) |
| Loss per share: | ||
| Basic and diluted | $ (0.41) | $ (0.65) |
| Weighted average shares outstanding: | ||
| Basic and diluted | 150,430 | 136,011 |
| (1) Amarin's costs include non-cash stock based compensation as well as warrant based compensation to former officers. Excluding non-cash stock and warrant based compensation, for 2013 and 2012 marketing, general and administrative expenses were $35,658 and $8,571 for 2013 and 2012, respectively, and research and development expenses were $21,024 and $3,964 respectively, for the same periods. | ||
| (2) Non-cash charges result from changes in the fair value of the warrant derivative liability. This liability is revalued at each reporting period and, upon exercise of warrants, is reclassified at fair value from liability to stockholders' equity. These warrants are valued using the Black-Scholes option pricing model, they are classified for accounting purposes as financial derivatives because, under certain circumstances, the exercise price of the warrants could increase. | ||
The following is a reconciliation of the non-GAAP financial measures used by Amarin to describe its financial results determined in accordance with United States generally accepted accounting principles (GAAP) An explanation of these measures is also included under the heading "Use of non-GAAP adjusted financial information" above.
| RECONCILIATION OF NON-GAAP LIABILITIES | ||
| Unaudited | ||
| March 31, 2013 | December 31, 2012 | |
| (in thousands) | ||
| Current Liabilities: | ||
| Accounts payable | $ 22,945 | $ 17,458 |
| Accrued interest payable | 4,646 | 2,520 |
| Deferred revenue | 2,865 | ----- |
| Accrued expenses and other liabilities | 11,935 | 5,224 |
| Total current liabilities | $ 42,391 | $ 25,202 |
| Long-Term Liabilities: | ||
| Warrant derivative liability | 49,011 | 54,854 |
| Exchangeable senior notes | 137,735 | 134,250 |
| Long-term debt | 85,873 | 85,153 |
| Long term debt redemption feature | 15,600 | 14,577 |
| Other long-term liabilities | 807 | 816 |
| Total liabilities – GAAP | $ 331,417 | $ 314,852 |
| Warrant derivative liability | (49,011) | (54,854) |
| Total liabilities – non GAAP | $ 282,406 | $ 259,998 |
| RECONCILIATION OF NON-GAAP NET LOSS | ||
| Unaudited | ||
| Three Months Ended March 31, | ||
| 2013 | 2012 | |
| Net loss for EPS1 – GAAP | $ (62,158) | $ (88,285) |
| Share based compensation expense | (4,874) | (3,874) |
| Warrant compensation income (expense) | 451 | (2,374) |
| Gain/(loss) on change in fair value of derivatives | 3,620 | (66,209) |
| Adjusted net loss for EPS1 – non GAAP | $ (61,355) | $ (15,828) |
| 1Basic and diluted | ||
| Loss per share: | ||
| Basic and diluted – non GAAP | $ (0.41) | $ (0.12) |
| Weighted average shares outstanding: | ||
| Basic and diluted | 150,430 | 136,011 |