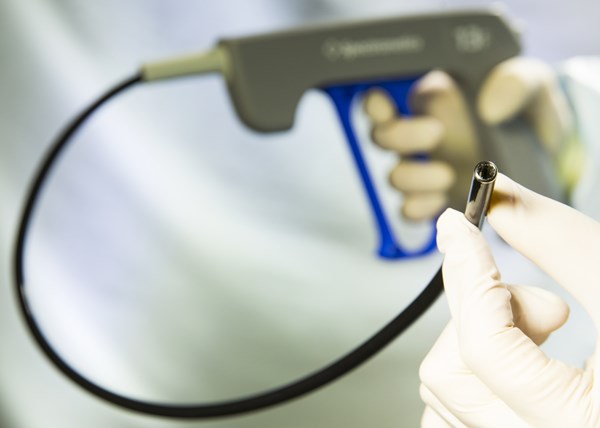
COLORADO SPRINGS, Colo., May 22, 2014 (GLOBE NEWSWIRE) -- The Spectranetics Corporation (Nasdaq:SPNC) today announced receipt of CE marking for the TightRail™ Rotating Dilator Sheath platform. The product combines unprecedented shaft flexibility and column strength to help physicians safely navigate the vasculature during cardiac lead extraction procedures. The announcement includes TightRail and a companion product: TightRail™ Mini Rotating Dilator Sheath. The Mini is designed to gain vascular access, dilating heavily fibrosed and calcified tissue. The new platform will be used to treat patients at facilities in Europe that are participating in Spectranetics' initial limited launch.
SightRail™ Manual Dilator Sheath, a partner platform, was awarded the CE mark in April and features visual indicators that show bevel orientation and tip alignment, supplementing fluoroscopy as a means to determine position and orientation
These new platforms represent Spectranetics' entry into the mechanical extraction device market, complementing the laser-based technology that established the company's leading position in cardiac lead extraction.
On the heels of completing TightRail's first in-patient case with Dr. Charles Love, Spectranetics debuted TightRail at the Heart Rhythm Society's 35th Annual Scientific Sessions in San Francisco earlier this month. Over 200 physicians participated in the "hands-on" Solutions Experience hosted by Spectranetics. TightRail will also headline the CardioStim conference in Nice, France, in June.
Donna Ford-Serbu, Senior Vice President, Sales and Marketing for Spectranetics Lead Management business unit, sees the mechanical extraction devices as an exciting addition to the company's portfolio of lead extraction solutions. "By providing a wider range of clinical solutions, we are responding to a global need for innovative extraction options," said Ford-Serbu. "Physicians are always looking for ways to serve their patient's needs – we are committed to providing tools that extend life and ensure physicians are successful at every turn."
There are over seven million implanted cardiac devices globally. Each year, about 750,000 additional patients are implanted with these devices worldwide, translating to 1.5 million leads. It is estimated that in 2014, roughly 400,000 patients will meet indications for a lead extraction procedure.
Photo attached.
About Spectranetics
Spectranetics develops, manufactures, markets and distributes single-use medical devices used in minimally invasive procedures within the cardiovascular system. The Company's products are sold in over 40 countries and are used to treat arterial blockages in the heart and legs, and the removal of pacemaker and defibrillator leads.
The Company's Vascular Intervention (VI) products include a range of laser catheters for ablation of blockages in arteries above and below the knee. The Company also markets support catheters to facilitate crossing of peripheral and coronary arterial blockages, and retrograde access and guidewire retrieval devices used in the treatment of peripheral arterial blockages, including chronic total occlusions. The Company markets aspiration and cardiac laser catheters to treat blockages in the heart.
The Lead Management (LM) product line includes excimer laser sheaths, mechanical sheaths and accessories for the removal of pacemaker and defibrillator cardiac leads.
For more information, visit www.spectranetics.com.
Safe Harbor Statement
This news release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. You can identify these statements because they do not relate strictly to historical or current facts. Such statements may include words such as "anticipate," "will," "estimate," "expect," "look forward," "strive," "project," "intend," "should," "plan," "believe," "hope," "enable," "potential," and other words and terms of similar meaning in connection with any discussion of, among other things, receipt of FDA approvals, future operating or financial performance, strategic initiatives and business strategies, clinical trials, regulatory or competitive environments, our intellectual property and product development. Such statements are based on current assumptions that involve risks and uncertainties that could cause actual outcomes and results to differ materially. You should not to place undue reliance on these forward-looking statements and note they speak only as of the date of this release.
These risks and uncertainties may include market acceptance of excimer laser atherectomy technology and our lead removal products, increasing price and product competition, increased pressure on expense levels resulting from expanded sales, marketing, product development and clinical activities, uncertain success of our strategic direction, dependence on new product development, uncertain success of or delays in our clinical trials, adverse results in any ongoing legal proceeding, or any legal proceeding in which we may become involved, adverse impact to our business of the healthcare reform and related legislation or regulations, including changes in reimbursements, continued or worsening adverse conditions in the general domestic and global economic markets and continued volatility and disruption of the credit markets, which affects the ability of hospitals and other health care systems to obtain credit and may impede our access to capital, intellectual property claims of third parties, availability of inventory from suppliers, adverse outcome of FDA inspections, the receipt of FDA approval to market new products or applications and the timeliness of any approvals, market acceptance of new products or applications, product defects, ability to manufacture sufficient volumes to fulfill customer demand, availability of vendor-sourced components at reasonable prices, unexpected delays or costs associated with any planned improvements to our manufacturing processes, and share price volatility due to the initiation or cessation of coverage, or changes in ratings, by securities analysts. For a further list and description of such risks and uncertainties that could cause our actual results, performance or achievements to materially differ from any anticipated results, performance or achievements, please see our previously filed SEC reports, including those risks set forth in our most recent Annual Report on Form 10-K. We disclaim any intention or obligation to update or revise any financial or other projections or other forward-looking statements, whether because of new information, future events or otherwise.
Photos accompanying this release are available at: http://www.globenewswire.com/newsroom/prs/?pkgid=25537