New York, USA, May 09, 2024 (GLOBE NEWSWIRE) -- Psoriasis Vulgaris Clinical Trial Pipeline Analysis Demonstrates 50+ Key Companies at the Horizon Expected to Transform the Treatment Paradigm, Assesses DelveInsight
The Psoriasis Vulgaris treatment market is expected to increase due to the increasing geriatric population across the globe. Advances in technology, heightened awareness of treatment options, and increased research and development efforts for Psoriasis Vulgaris treatments are all poised to boost market expansion.
DelveInsight’s 'Psoriasis Vulgaris Pipeline Insight 2024' report provides comprehensive global coverage of pipeline psoriasis vulgaris therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the psoriasis vulgaris pipeline domain.
Key Takeaways from the Psoriasis Vulgaris Pipeline Report
- DelveInsight’s psoriasis vulgaris pipeline report depicts a robust space with 50+ active players working to develop 55+ pipeline therapies for psoriasis vulgaris treatment.
- Key psoriasis vulgaris companies such as Xencor, Ono Pharmaceutical Co. Ltd, Boehringer Ingelheim, GC Cell Corporation, Soligenix, Cantargia AB, Huabo Biopharm, Huaota Biopharmaceuticals, Bio-Thera Solutions, Hangzhou Highlightll Pharmaceutical, Sanofi, Johnson & Johnson Innovative Medicine, Artax Biopharma, Suzhou Suncadia Biopharmaceuticals Co., Ltd., Arxx Therapeutics, SFA Therapeutics, InventisBio, Beijing InnoCare Pharma Tech Co., LtdSun Pharmaceutical Industries Limited, E-nitiate Biopharmaceuticals, Meiji Pharma, and others are evaluating new psoriasis vulgaris drugs to improve the treatment landscape.
- Promising psoriasis vulgaris pipeline therapies such as XmAb564, BAT-2306, ONO-4685, BI 765250, CT303, SGX302, CAN10, HB0017, HOT-3010, TLL018, SAR441566, JNJ-2113, AX-158, SHR-1314, AX-202, SFA002, D-2570, ICP-488, SCD-044, QY101, ME3183, and others are under different phases of psoriasis vulgaris clinical trials.
- In March 2024, Alumis Inc. announced the presentation of positive clinical data from a Phase II clinical trial of ESK-001, a highly selective allosteric tyrosine kinase 2 (TYK2) inhibitor, for the treatment of patients with moderate-to-severe plaque psoriasis. These data were presented during a late-breaking session at the American Academy of Dermatology (AAD) Annual Meeting being held March 8-12 in San Diego, California.
- In February 2024, Biocon Biologics announced that the Company has signed a settlement and license agreement with Janssen Biotech Inc., and Johnson & Johnson (collectively known as Janssen) that clears the way to commercialize its Bmab 1200, a proposed biosimilar to Stelara, in the United States of America.
- In February 2024, Johnson & Johnson announced the publication in the New England Journal of Medicine (NEJM) of the Phase IIb FRONTIER 1 trial results for JNJ-2113. JNJ-2113 is the first and only investigational targeted oral peptide inhibitor designed to block the IL-23 receptor.
- In November 2023, Bio-Thera Solutions, Ltd. announced results from the Phase III study of BAT2206, a proposed biosimilar referencing Stelara (ustekinumab). The primary endpoint of this study was improvement from baseline in Psoriasis Area and Severity Index (PASI) score to Week 12, demonstrating BAT2206 is highly similar with Stelara® in patients with moderate to severe plaque psoriasis.
- In February 2023, Innovent Biologics announced that the first patient with moderate-to-severe plaque psoriasis had been successfully dosed in a Phase III clinical trial (CLEAR) of picankibart (IBI112), a recombinant anti-interleukin 23p19 subunit (IL23p19) antibody injection.
- In October 2023, Meiji Seika Pharma Co., Ltd. announced that positive findings from the Phase II clinical trial of ME3183, a novel highly-potent selective phosphodiesterase-4 (PDE4) inhibitor, in patients with plaque psoriasis conducted in the United States and Canada (NCT05268016) were presented on 12 October at the European Association of Dermatology and Venereology (EADV) Congress 2023 held in Berlin.
Request a sample and discover the recent advances in psoriasis vulgaris treatment drugs @ Psoriasis Vulgaris Pipeline Report
The psoriasis vulgaris pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage psoriasis vulgaris drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the psoriasis vulgaris clinical trial landscape.
Psoriasis Vulgaris Overview
Psoriasis vulgaris is a long-lasting skin condition marked by the quick renewal of the outer skin layer, leading to the buildup of thick scales in areas prone to frequent injury or irritation. It is also referred to as chronic plaque psoriasis or plaque psoriasis, characterized by raised, reddish skin patches covered with silvery scales. This type of psoriasis is the most prevalent, affecting around 80-90% of individuals with the condition. Psoriasis itself is a diverse and lifelong skin disorder that appears in various forms such as plaque, flexural, guttate, pustular, or erythrodermic.
The development of psoriasis involves excessive skin cell growth and changes in how keratinocytes mature. Its root cause is a combination of genetic, immunological, and environmental factors. Specific environmental factors are believed to disturb the skin's immune system balance in people genetically predisposed to the condition. Some medications, like angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), antimalarials, lithium, and nonsteroidal anti-inflammatory drugs, have been linked to the onset or worsening of psoriasis.
Doctors typically diagnose psoriasis vulgaris by physically examining the skin, scalp, and nails for characteristic signs. They may inquire about the patient's health, medical background, family history, recent illnesses, and significant stress. Occasionally, a small skin sample might be needed for microscopic examination to exclude other skin conditions resembling psoriasis and to identify the specific type. However, a skin biopsy is rarely necessary for diagnosis.
Find out more about psoriasis vulgaris treatment drugs @ Drugs for Psoriasis Vulgaris Treatment
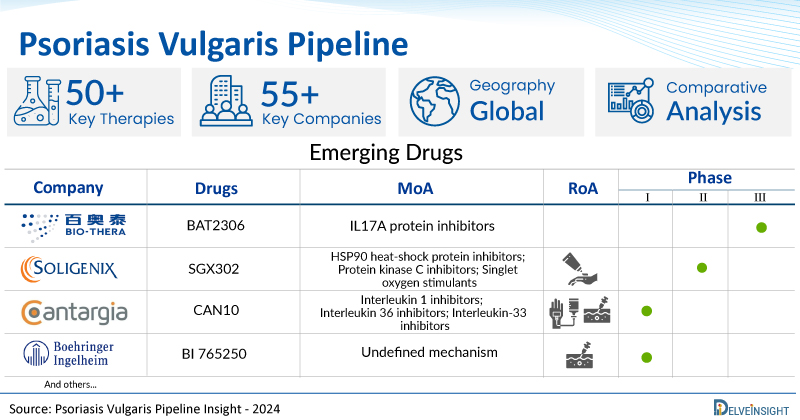
A snapshot of the Psoriasis Vulgaris Pipeline Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| BAT2306 | Bio-Thera Solutions | Phase III | IL17A protein inhibitors | Subcutaneous |
| SGX302 | Soligenix | Phase II | HSP90 heat-shock protein inhibitors; Protein kinase C inhibitors; Singlet oxygen stimulants | Topical |
| CAN10 | Cantargia AB | Phase I | Interleukin 1 inhibitors; Interleukin 36 inhibitors; Interleukin-33 inhibitors | Intravenous, Subcutaneous |
| XmAb564 | Xencor | Phase I | Interleukin-2 replacements; Regulatory T-lymphocyte stimulants | Subcutaneous |
| BI 765250 | Boehringer Ingelheim | Phase I | Undefined mechanism | Intravenous, Subcutaneous |
Learn more about the emerging psoriasis vulgaris pipeline therapies @ Psoriasis Vulgaris Clinical Trials
Psoriasis Vulgaris Therapeutics Assessment
The psoriasis vulgaris pipeline report proffers an integral view of the psoriasis vulgaris emerging novel therapies segmented by stage, product type, molecule type, mechanism of action, and route of administration.
Scope of the Psoriasis Vulgaris Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Parenteral, Intravenous, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Monoclonal Antibody, Peptides, Polymer, Small molecule, Gene therapy
- Therapeutics Assessment By Mechanism of Action: IL17A protein inhibitors, Cell death stimulants, HSP90 heat-shock protein inhibitors, Immunosuppressants, Photosensitisers, Protein kinase C inhibitors, Singlet oxygen stimulants, Interleukin 1 inhibitors, Interleukin 36 inhibitors, Interleukin-33 inhibitors, Interleukin-2 replacements, Regulatory T-lymphocyte stimulants
- Key Psoriasis Vulgaris Companies: Xencor, Ono Pharmaceutical Co. Ltd, Boehringer Ingelheim, GC Cell Corporation, Soligenix, Cantargia AB, Huabo Biopharm, Huaota Biopharmaceuticals, Bio-Thera Solutions, Hangzhou Highlightll Pharmaceutical, Sanofi, Johnson & Johnson Innovative Medicine, Artax Biopharma, Suzhou Suncadia Biopharmaceuticals Co., Ltd., Arxx Therapeutics, SFA Therapeutics, InventisBio, Beijing InnoCare Pharma Tech Co., LtdSun Pharmaceutical Industries Limited, E-nitiate Biopharmaceuticals, Meiji Pharma, and others
- Key Psoriasis Vulgaris Pipeline Therapies: XmAb564, BAT-2306, ONO-4685, BI 765250, CT303, SGX302, CAN10, HB0017, HOT-3010, TLL018, SAR441566, JNJ-2113, AX-158, SHR-1314, AX-202, SFA002, D-2570, ICP-488, SCD-044, QY101, ME3183, and others
Dive deep into rich insights for new drugs for psoriasis vulgaris treatment, visit @ Psoriasis Vulgaris Drugs
Table of Contents
| 1. | Psoriasis Vulgaris Pipeline Report Introduction |
| 2. | Psoriasis Vulgaris Pipeline Report Executive Summary |
| 3. | Psoriasis Vulgaris Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Psoriasis Vulgaris Clinical Trial Therapeutics |
| 6. | Psoriasis Vulgaris Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Psoriasis Vulgaris Pipeline: Late-Stage Products (Phase III) |
| 8. | Psoriasis Vulgaris Pipeline: Mid-Stage Products (Phase II) |
| 9. | Psoriasis Vulgaris Pipeline: Early-Stage Products (Phase I) |
| 10. | Psoriasis Vulgaris Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Psoriasis Vulgaris Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Psoriasis Vulgaris Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the psoriasis vulgaris pipeline therapeutics, reach out @ Psoriasis Vulgaris Treatment Drugs
Related Reports
Psoriasis Vulgaris Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key psoriasis vulgaris companies, including Circassia Limited, Bioskin GmbH, LEO Pharma, CMIC Co. Ltd, Eisai Inc., Creabilis SA, Theramab LLC, Glenmark Pharmaceuticals Ltd, Philips Electronics Nederland BV, Kadmon Corporation LLC, Bristol-Myers Squibb, AstraZeneca, Parexel, Delenex Therapeutics AG, Sienna Biopharmaceuticals, Galderma R&D., Janssen Research & Development LLC, MC2 Therapeutics, Tigermed Consulting Co., Ltd, Manhattan Pharmaceuticals, Allergan, Pfizer, Sun Pharmaceutical Industries Limited, Applied Biology Inc., Eli Lilly and Company, among others.
Psoriasis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key psoriasis companies, including Mylan, Biocad, Bristol-Myers Squibb, Celltrion, Coherus BioSciences, Janssen Pharmaceuticals, Can-Fite Biopharma, Arcutis Biotherapeutics, Amgen, Iltoo Pharma, GlaxoSmithKline, Galectin Therapeutics, Evelo Biosciences, Galderma, BioMimetix JV, Menlo Therapeutics Inc., Aristea Therapeutics, UNION Therapeutics, MetrioPharm, Sienna Biopharmaceuticals, among others.
Plaque Psoriasis Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key plaque psoriasis companies including Suzhou Zelgen Biopharmaceuticals, Sun Pharmaceutical Industries Limited, GC Cell Corporation, among others.
Chronic Plaque Psoriasis Pipeline
Chronic Plaque Psoriasis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key chronic plaque psoriasis companies, including Jiangsu Hengrui Medicine, Akeso Biopharma, Suzhou Zelgen Biopharmaceuticals, among others.
Mild to Moderate Plaque Psoriasis Market
Mild to Moderate Plaque Psoriasis Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key mild to moderate plaque psoriasis companies including Suzhou Zelgen Biopharmaceuticals, Acrutis Biotherapeutics, AnaptysBio, Evelo Biosciences, BMS, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
