- Emergex has entered into an agreement with DEKA Research & Development (‘DEKA’) to assess the compatibility of Emergex’s T cell-priming set-point immunotherapeutic candidates with DEKA’s Intradermal Therapeutic Applicator. The collaboration builds on previous data showing satisfactory loading and stability of Emergex’s therapeutic candidates when incorporated with the Intradermal Therapeutic Applicator.
- The collaboration also covers completion of a preclinical study to compare the pharmacokinetics of [i] Emergex’s Dengue treatment candidate by syringe-based microneedle technology, which have been used in previous preclinical and clinical Emergex studies, with [ii] DEKA’s Intradermal Therapeutic Applicator.
- Pending preclinical study results, the agreement allows for the possibility of advancing the Intradermal Therapeutic Applicator as an administration device for Emergex’s pipeline of treatments for viral diseases.
ABINGDON, United Kingdom, Jan. 16, 2024 (GLOBE NEWSWIRE) -- Emergex Vaccines Holding Limited (‘Emergex’, or the ‘Company’), a clinical-stage biotechnology company addressing major global infectious diseases through the development of synthetic T cell-priming set-point immunotherapy-based treatment candidates, today announced that the Company has entered into an agreement with DEKA to assess the compatibility of Emergex’s immunotherapeutic candidates with the DEKA Intradermal Therapeutic Applicator.
The Intradermal Therapeutic Applicator incorporates hollow microneedle technology into a convenient self-administration device. This agreement builds on previous data showing satisfactory loading and stability of Emergex’s product candidates when incorporated into the Intradermal Therapeutic Applicator.
Laurens Rademacher, Chief Technology Officer at Emergex, added: “We are delighted to announce this important collaboration with DEKA. Intradermal/Epidermal delivery is critical to the mechanism of our T cell-priming product platform. The use of microneedles, which have been utilized in all our previous preclinical and clinical work, paired with the ease-of-use of the Intradermal Therapeutic Applicator is a perfect complement to our own technologies.”
The agreement covers completion of a preclinical pharmacokinetic study to compare the delivery of Emergex’s DengueTcP1 product candidate by syringe-based microneedle technology versus the DEKA Intradermal Therapeutic Applicator. Pending completion, if promising data is obtained, the agreement allows for the possibility of advancing DEKA’s Intradermal Therapeutic Applicator as a device for administration of Emergex’s T cell-priming set-point immunotherapeutic product candidates in the pipeline.
Dean Kamen, Founder and President, of DEKA Research & Development, commented: “The partnership with Emergex is a meaningful step forward in patient therapeutic delivery. The combined technologies of our novel intradermal therapeutic delivery platform along with Emergex’s products can open a new route to intradermal drug administration in a patient friendly, easy-to-use, painless way. We are excited by the potential of our combined technologies in saving numerous lives.”
About DEKA Intradermal Therapeutic Applicator
DEKA’s technology leverages hollow microneedles that offer a unique design with flexible features for efficient therapeutic delivery, self-administration and minimal pain. With a simple push of the top button on the device, the petal design spreads outward, thereby stretching the skin and enabling a consistent insertion of the microneedles into the skin. The continued downward motion then activates the trigger that applies pressure to the reservoir to expel the therapeutic agent into the intradermal region. Numerous advantages of this device include self-administration, dose-sparing and less overall waste than conventional needles.
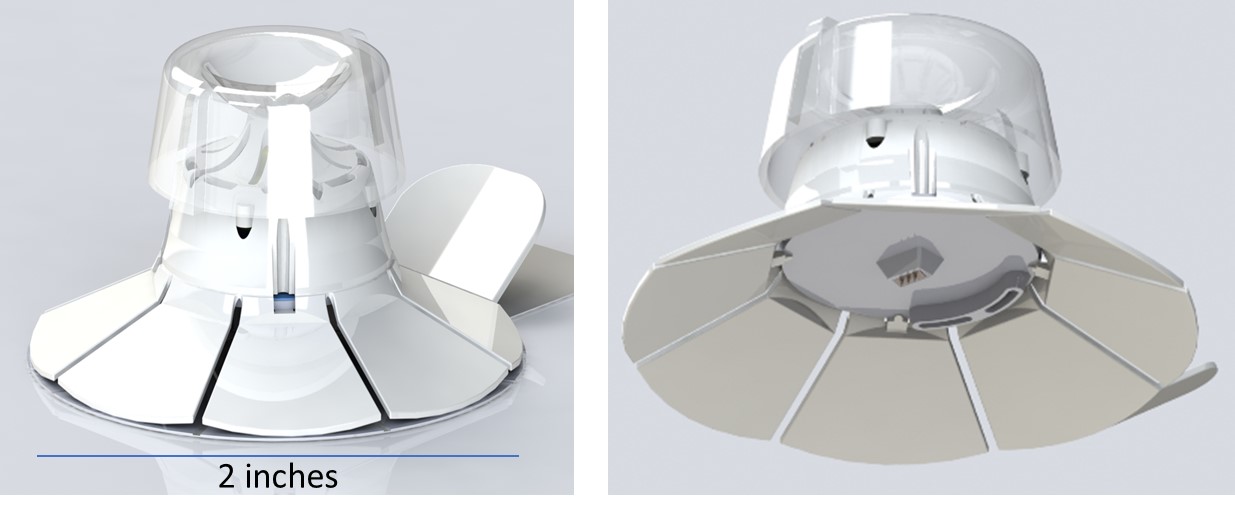
About DEKA Research and Development
Based in Manchester, NH, DEKA Research & Development Corp. is a technology development organization. The company was founded in 1982 by Dean Kamen.
Find out more online at www.dekaresearch.com.
Visit our LinkedIn page for updates.
About Emergex
Emergex is a clinical-stage, privately held biotechnology company, headquartered in Abingdon, UK, with an operating subsidiary in Doylestown, Pennsylvania and a GMP manufacturing facility in Fremont, CA, USA. The Company is pioneering the development of 100% synthetic, T cell-priming immune set-point vaccine candidates designed to mimic the body’s natural T cell immune response to destroy and to clear pathogen-infected cells, using cytopathic or non-cytopathic mechanisms, in order to protect against some of the world’s most urgent health threats. The candidates are also specifically designed for administration using novel micro-needles via skin immunisation into the epidermal layer, intended to reduce the burden and logistics associated with conventional preventative measures. Emergex’s first indications pursued are against infectious diseases: [i] viral infectious diseases, amongst which are Betacoronaviruses, Dengue Fever and Universal Influenza (including pandemic influenza) candidates, as well as [ii] intra-cellular bacterial infectious disease, such as tularemia caused by Francisella tularensis. Emergex has a growing proprietary pipeline of innovative candidates with potential to deliver rapid, broad (strain and variant agnostic) and long-lasting prevention to reduce serious illness associated with infectious diseases.
Find out more online at www.emergexvaccines.com.
Visit our LinkedIn page or Twitter account for updates.
For further information, please contact:
| Emergex Storme Moore-Thornicroft, Executive Director Phone: +44 (0) 1235 527589 Email: smt@emergexvaccines.com | Media Inquiries Rachelle Babb, Senior Account Executive Phone: +1 (929) 325-7559 Email: rachelle.babb@russopartnersllc.com |
1 Trademark application submitted
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/1fc26fd8-c29e-41b9-8d86-6564c43f916c
