Long-Term Data Supports Three-Year Durability
Innovative Design Allows for Only Tailored BPH Approach that Preserves Natural Anatomy
SOUTH SAN FRANCISCO, Calif., Dec. 11, 2025 (GLOBE NEWSWIRE) -- Zenflow, Inc. announced today the U.S. Food and Drug Administration (FDA) approval of the Zenflow Spring® Implant and Delivery System for the treatment of symptoms associated with benign prostatic hyperplasia (BPH), also known as enlarged prostate. The Zenflow Spring Implant and Delivery System is a novel, first-line interventional therapy (FIT) that provides urologists and their patients with a minimally invasive option that has proven long-term durability and unique safety advantages, including no post-procedure catheterization and the ability to reverse treatment if desired.
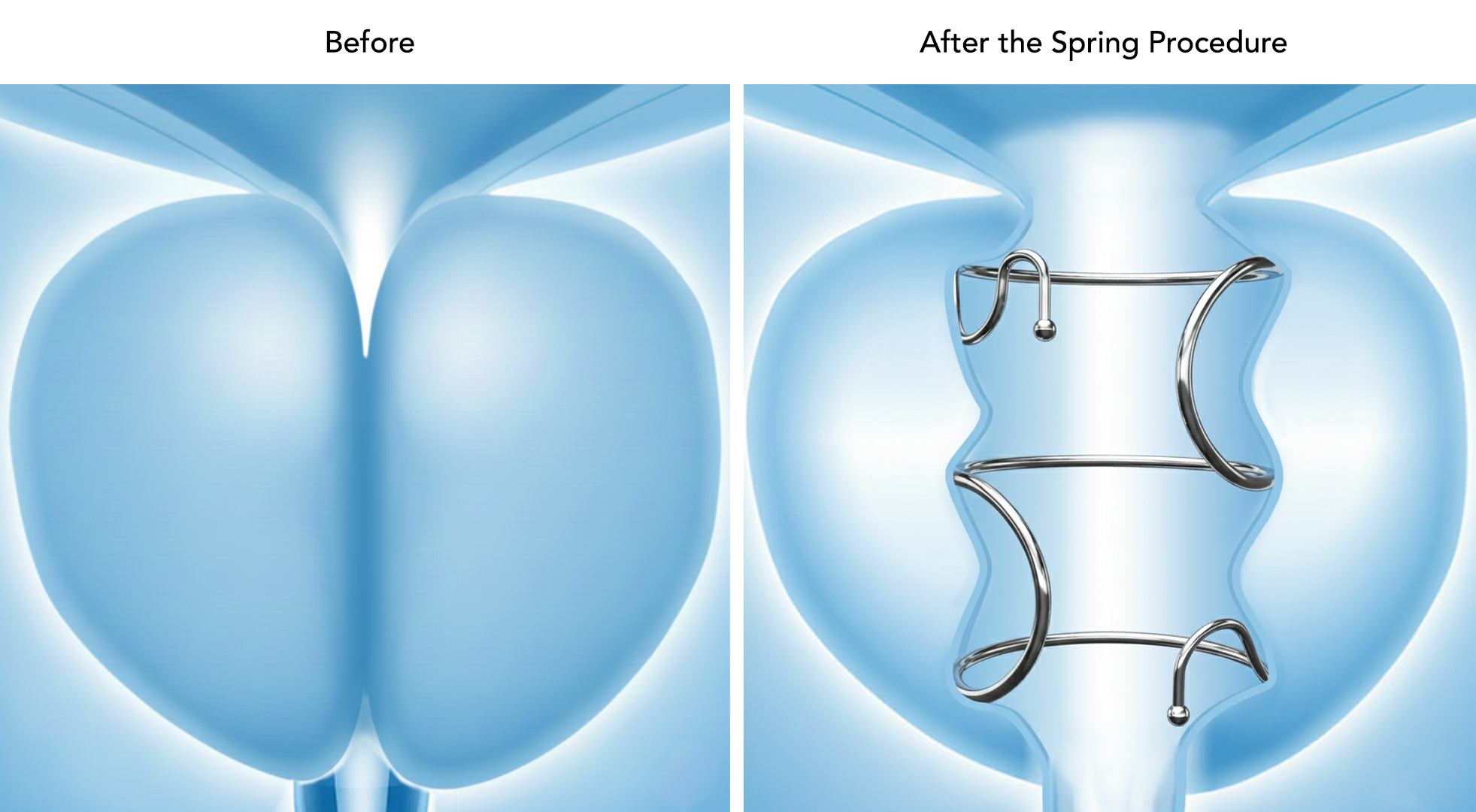
Designed with the patient experience in mind, the Zenflow Spring Implant and Delivery System opens the urethra while preserving natural anatomy through a proprietary small spring-like implant. The Spring is the only FDA-approved FIT in a range of lengths and diameters, allowing for patient personalization. It is made from a superelastic shape-memory material and delivered in an outpatient setting using the recently FDA-cleared Zenflow Spring® Scope – a single-use, flexible cystoscope. This approval represents the very first FDA-approved BPH therapy delivered via a flexible cystoscope, which enhances patient comfort and recovery. Unlike other BPH interventions, the Zenflow Spring Implant and Delivery System eliminates burning, cutting, piercing, or resecting tissue. Zenflow’s unique design also offers complete reversibility without fear of complications commonly associated with other minimally invasive BPH treatments.
“Today’s FDA approval marks the culmination of years of innovation,” said Shreya Mehta, CEO of Zenflow. “Urologists and patients alike have long sought a flexible, gentle solution that combines the reversibility of medication with the long-term durability of an interventional approach. For the first time, men living with BPH symptoms have a choice that offers relief without compromise. We are excited to bring this new therapy to market.”
“First-line interventional therapy with the Zenflow Spring Implant and Delivery System provides effective symptom relief with rapid recovery, minimal side effects, and preserved treatment adaptability,” said Dean Elterman, M.D., Associate Professor at the University of Toronto and an attending urologist at the University Health Network. “The FDA’s approval of this novel treatment offers hope to men living with BPH because it simultaneously improves disruptive BPH symptoms while preserving sexual function.”
Key Data Highlights Supporting FDA Approval
The BREEZE pivotal study supporting FDA approval demonstrated remarkable outcomes and met all primary safety and effectiveness endpoints in the intended use population. Highlights include:
- Effectiveness: Responder rate was 60% (compared to 33% for sham) at one year, with a mean International Prostate Symptom Score (IPSS) improvement of 37% exceeding the pre-specified clinical success threshold of 30%.
- Safety: Best-in-class safety outcomes demonstrating that 99% of men did not need a post-procedure catheter, a 0% device-related Serious Adverse Event (SAE) rate, and an overall related AE rate of 13%.
- Patient Satisfaction: 71% of subjects agree that the Zenflow Spring Implant and Delivery System improved their urinary symptoms, and 66% said that they would recommend this treatment to others.
- Sexual Health: No deterioration in sexual function, including erectile or ejaculatory function.
Long-Term Data Support Effective, Durable, and Safe Use
Notably, the pivotal study supporting FDA approval also aligns with prior pilot studies that demonstrate durability through three years. Data from patients in the ZEST trials demonstrated statistically significant and sustained reductions in IPSS from baseline at all assessment time points. In the pilot studies, the responder rate (proportion of subjects with ≥ 30% improvement in IPSS) was 74% at three years, similar to 75% at one year and 70% at two years, with no deterioration in erectile or ejaculatory function over three years.
About Benign Prostatic Hyperplasia
Found in the male reproductive system, the prostate is a small gland that helps make semen and surrounds the tube that carries urine out of the body called the urethra. As men get older, the prostate can grow larger, which is called benign prostatic hyperplasia (BPH) or enlarged prostate. This growth can press on the urethra, making it harder to urinate and leading to symptoms that disrupt quality of life, including difficulty starting or fully emptying the bladder, a weak or interrupted stream, increased frequency and urgency, nighttime urination, and discomfort. An estimated 32 million men over the age of 60 in the United States and 79 million men worldwide live with BPH.1 More than 50% of men are impacted by BPH symptoms by age 50, 70% of men by age 70, and 90% of men by age 80.2
About Zenflow
Zenflow, Inc. is a medical device company dedicated to transforming urologic care with superior design and patient-focused innovation. The innovative Zenflow Spring® Implant and Delivery System (Zenflow Spring® Scope, Implant, and Delivery system) was designed to optimize the patient experience for men living with benign prostatic hyperplasia (BPH) symptoms. For more information, visit www.zenflow.com, and follow Zenflow on LinkedIn and X for the latest updates.
MEDIA CONTACT:
Rebecca Novak
Rebecca@RNTCommunications.com
References
1. Ye, Z., Wang, J., Xiao, Y. et al. Global burden of benign prostatic hyperplasia in males aged 60–90 years from 1990 to 2019: results from the global burden of disease study 2019. BMC Urol 24, 193 (2024). https://doi.org/10.1186/s12894-024-01582-w https://link.springer.com/article/10.1186/s12894-024-01582-w#citeas
2. Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7 Suppl 9(Suppl 9):S3-S14. PMID: 16985902; PMCID: PMC1477638. https://pmc.ncbi.nlm.nih.gov/articles/PMC1477638/#B1
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/91fb72e9-3f6d-42e2-b320-e110f0c0bdaa
