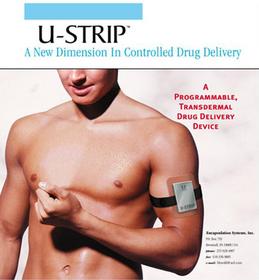
-- Transdermal Patch is specially designed to hold the target drug.
-- Sonic Applicator Module is a programmable ultrasonic transmitter that
fits directly above the patch. The ultrasonic transmitter can be
programmed to deliver a constant or pulse of ultrasonic energy, through the
transdermal patch. Ultrasound from the Sonic Applicator Module pushes the
drug (insulin) contained within the patch through the skin.
-- A rechargeable battery system is located within the strap, which holds
the ultrasonic transmitter over the patch.
U-STRIP ADVANTAGES:
The U-Strip offers a number of advantages to both the patient and to
medical professionals which include:
-- Non-Invasive - No needles are required with this totally non-invasive
drug delivery system.
-- Safe and effective - U-Strip controls the patient's medication so
there is less change of over/under dosage.
-- Direct bloodstream absorption - This on-demand delivery system
bypasses the stomach and the intestine where drug potency can be destroyed.
-- Individualized drug regimen - Medical professionals can easily
individualize drug regimen tailored for each patient's needs.
-- Easy to Follow - The automatic drug delivery ensures heightened
compliance for pediatric and elderly patients, because there is nothing to
remember and no schedules to follow. Patients simply "Set it and Forget it"
and ensure their drug regimen is more easily followed.
-- Additional Pharmaceutical Applications - The U-Strip technology may
open the door to a number of advanced pharmaceutical delivery applications
for other chronic medical conditions.
BACKGROUND:
The National Diabetes Information Clearinghouse reports that there are
"20.8 million children and adults in the United States, or 7% of the
population, who have diabetes," a disease in which the body does not
produce or properly use insulin, the hormone needed to convert sugar,
starches and other food into energy needed for daily life. There are two
major types of diabetes, Type 1, which results from the body's failure to
produce insulin, and Type 2, which results from insulin resistance (a
condition in which the body fails to properly use insulin). Most Americans
who are diagnosed with diabetes have Type 2 diabetes.
Treatment for diabetes varies depending on the type of diabetes one has
been diagnosed with, ranging from insulin injections/pumps, which are
sometimes painful, to daily oral drug regimens. For most diabetic patients
treatment methods and drug delivery systems can often be cumbersome and
frustrating.
THE FUTURE - U-STRIP INSULIN SYSTEM
The U-Strip™ is a patent pending drug delivery system in Phase-2
clinical trials that is not yet approved for human use by the FDA.
However, the development of a programmable, portable, ultrasonic instrument
will extend the number and types of drugs that can be given with the
transdermal patch.
"For almost 20 years we have been the leader in specialty controlled
release products for use in food, pharmaceuticals and medical devices,"
adds Redding. "However, the U-Strip Insulin System marks a milestone in
our company's history by opening the door to a number of advanced
pharmaceutical delivery applications and demonstrates our investment and
ongoing commitment in research and development."
ABOUT ENCAPSULATION SYSTEMS INC:
Founded in 1988, Encapsulation Systems Inc. www.encsys.com (ESI) is a
private company specializing in the development and commercialization of
specialty controlled release products for use in the food, pharmaceutical
and medical device fields. A major innovator of controlled delivery
systems since its inception, ESI has 11 patent applications pending in the
United States and internationally and has claimed 57 new inventive concepts
regarding the U-Strip Technology.
B-Roll includes visuals of the U-Strip Insulin patch and interviews with:
Bruce K. Redding, creator of U-Strip Insulin Patch and president/CEO,
Encapsulation Systems.
Rex Kessler, M.D. Endocrinologist, Kessler Research Institute, Principal
Investigator U-Strip Human Pilot Trial 2
Ms. Bo Michniak-Kohn, Ph.D., Associate Professor Pharmaceutics, Rutgers,
State University of New Jersey, U-Strip Scientific Advisory Board Member
FOR B-ROLL - Please call Nancy Tamosaitis, Vorticom Inc. 212.532.2208 or
email nancyt@vorticom.com for b-roll. The launch press release announcing
the company's human pilot trial results is also available by calling
212.532.2208.
Contact Information: Media Contacts: Nancy Tamosaitis 917.371.4053 (mobile) 212.532.2208 (office) Carolyn Marquez 347.885.6722 (mobile)