CHARLOTTESVILLE, Va., Sept. 13, 2021 (GLOBE NEWSWIRE) -- RIVANNA®, developers of world-first, imaging-based medical solutions, today announced the results of a prospective, double-blind study demonstrating that the Accuro® Epidural Guidance device with SpineNav3D™ technology automatically identifies and measures epidural space depth with the same accuracy as standard ultrasound in obese pregnant women. The study, published in the Open Journal of Anesthesiology, included 45 pregnant women with a body mass index (BMI) of ≥ 30 kg/m2 requesting an epidural for labor analgesia or neuraxial anesthesia for elective cesarean delivery.
Many patients gain a significant amount of weight during pregnancy, thus satisfying the requirement for obesity: BMI > 30 kg/m2. Obesity complicates the anatomical landmarks needed for epidural space localization, making the identification of bony landmarks by palpation difficult. Additional body fat, combined with pregnancy-induced softening of joint ligaments and muscle tissue, may increase technical challenges in identifying the epidural space using the loss-of-resistance technique.
Standard ultrasonography has been proven helpful in predicting depth to the epidural space and reliably determining the skin puncture site in obese patients. However, widespread use of neuraxial ultrasound may be limited by technical expertise, difficulty obtaining and interpreting the images or accessibility to ultrasound equipment.
Accuro with AI-Enabled SpineNav3D™ Image Recognition technology automatically identifies bony landmarks, provides real-time confirmation of interspaces within the lumbar ultrasound image and automatically annotates the epidural depth measurement, thereby reducing difficulty interpreting ultrasound images.
Prior studies show that Accuro can accurately determine the depth to the epidural space, correctly identifying the interspace in normal-weight women receiving labor epidural analgesia. Moreover, studies show that Accuro can determine epidural depth in term-pregnant women with accuracy equivalent to standard ultrasound.
An important finding from this study is that Accuro, which requires less operator interpretation of ultrasound images, provides epidural space depth estimates with accuracy equivalent to standard ultrasound in obese pregnant women. Quoted from the study, "[Accuro] requires less operator interpretation of ultrasound images, while offering automated real-time identification of interspaces and epidural depth and this feature may be useful in many clinical settings where there is a lack of equipment, or of a physician's adequate training or the need for rapid bedside use."
"Ongoing clinical trials and research prove the device's efficacy and benefits in promoting procedure safety, efficiency, cost-cutting and patient satisfaction," commented Will Mauldin, PhD, co-founder and CEO of RIVANNA. "Previous studies confirm that Accuro significantly enhances the accuracy of spinal anesthesia placement compared to the traditional palpation-based approach—irrespective of provider experience level, type of neuraxial anesthesia performed and amount of prior training with Accuro. We are pleased with the breadth of clinical evidence solidifying Accuro as an effective, easy-to-learn tool such that anesthesia providers with even minimal ultrasound experience can take advantage of its powerful image recognition technology."
Conducted at San Gerardo Hospital, Monza, Italy, the trial is registered at Clinical Trials.gov (NCT 04395573).
Media Contact: Vicki Brothers | vbrothers@rivannamedical.com
Related Images
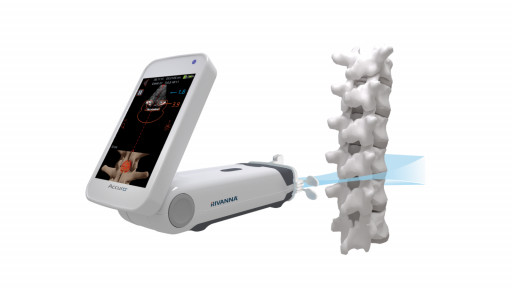
Image 1: Accuro's SpineNav3D Epidural Guidance
This content was issued through the press release distribution service at Newswire.com.
Attachment
