PRINCETON, N.J., Nov. 03, 2022 (GLOBE NEWSWIRE) -- PharmaNest, an emerging leader in quantitative Digital Pathology and Artificial Intelligence, announces today that it will present, alongside with its collaborators, three abstracts at this year's Liver Meeting | AASLD Conference, Nov. 4-8, 2022 in Washington, D.C. The abstracts highlight the utility and performance of FibroNest, the first multi-vendor quantitative digital Pathology image analysis and translational platform for the assessment of the severity and progression of Fibrosis and disease activity in NASH and Cirrhosis, and other fibrotic conditions.
"The automated quantification of the phenotype of fibrosis severity from the same slides reviewed by pathologists offers a robust and scalable method to generate continuous scores across the continuum of NAFLD, NASH and Cirrhosis. Thanks to its high detection thresholds, the FibroNest method can measure faint changes and specific traits of disease regression induced by candidate drugs," said Mathieu Petitjean, CEO and founder of PharmaNest.
The first two abstracts are retrospective analysis of liver biopsies from the LiFT Phase 2 trial of LPCN1144 (NCT04134091). They show (i) how FibroNest's continuous fibrosis score detects drug effects that are missed by conventional histological methods and (ii) that the quantification of the anti-steatosis effect of LPCN1144 is similar whether using MRI-PDFF, FibroNest Steatosis scores, or histological steatosis grades (in partnership with Lipocine, Inc).
A third abstract describes the performance of the FibroNest fibrosis score along the Cirrhosis spectrum (N=100) and demonstrates how the phenotypic nature of this score reduces the intra-("geographic") coefficient of variability in liver biopsies from previously reported values of 47% down to 16% in average (in partnership with Mount Sinai University).
The specific details of these abstracts are available on PharmaNest website at fibronest.com/science.
As a multi-vendor platform, FibroNest is compatible with all kinds of digital image formats including conventional digital pathology-stained slides (Picro Sirius Red, Trichrome or Antibody stains for fibrosis) acquired by FDA-approved digital pathology scanners and other digital microscopes. It is a Research-Use-Only tool for now.
Contact Information:
Mathieu Petitjean
CEO
info@pharmanest.com
(609) 375 2003
Related Images
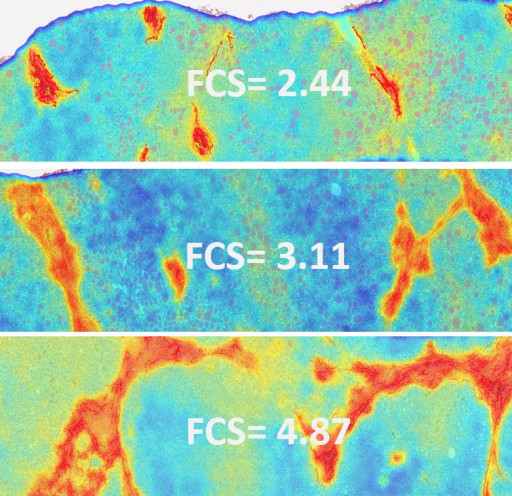
Image 1: FibroNest Ai Scores
FibroNest's Fibrosis Severity score in Liver Biopsies of NASH patients
This content was issued through the press release distribution service at Newswire.com.
Attachment
