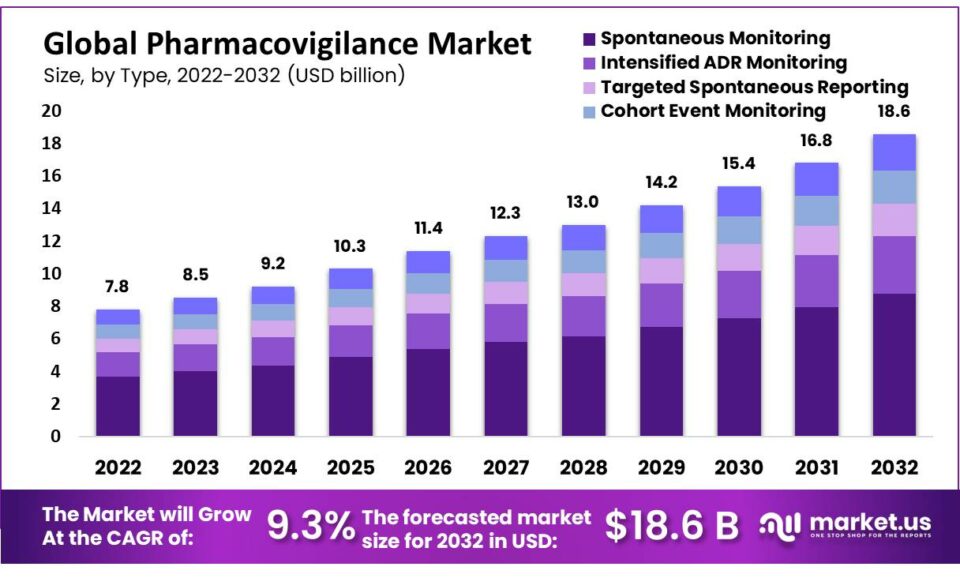
New York, Dec. 11, 2023 (GLOBE NEWSWIRE) -- According to Market.us, The global Pharmacovigilance Market is anticipated to be USD 18.6 billion by 2032. It is estimated to record a steady CAGR of 9.3% in the Forecast period 2023 to 2032. It is likely to total USD 9.2 billion in 2024.
Pharmacovigilance refers to the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. The Pharmacovigilance Market encompasses various products and services aimed at ensuring the safety of pharmaceuticals and healthcare products throughout their life cycle. The pharmacovigilance market has grown significantly in response to increased regulatory requirements and the need for ensuring patient safety. As new drugs are developed and introduced into the market, ongoing vigilance is crucial to identifying and addressing any unexpected adverse effects or safety concerns. This market plays a vital role in maintaining the overall safety and effectiveness of pharmaceutical and healthcare products.
Request Sample Report and Drive Impactful Decisions: https://market.us/report/pharmacovigilance-market/request-sample/
Key Takeaway
- Market Growth: The global pharmacovigilance market is poised to reach USD 19 billion by 2032, exhibiting a robust compound annual growth rate (CAGR) of 9.3%.
- Chronic Disease Impact: The escalating prevalence of chronic diseases is a major catalyst, propelling increased drug consumption and a heightened demand for pharmacovigilance services.
- Spontaneous Reporting: Holding the largest market share, spontaneous reporting is favored for its data simulation capabilities and the ability to facilitate effective drug comparisons.
- Cohort Event Monitoring: This segment is experiencing a surge in popularity driven by the widespread adoption of data mining techniques and enhanced surveillance practices involving both new and established medicines.
- Targeted Spontaneous Reporting: Anticipated as the fastest-growing segment, driven by government initiatives promoting diverse reporting methods to enhance drug safety monitoring.
- Electronic Health Records (EHR): The growing utilization of electronic health records post-hospital discharge is fueling the expansion of this segment, particularly in identifying and mitigating risks associated with drug use.
- Phase IV Dominance: Post-marketing surveillance in Phase IV is deemed crucial for monitoring and mitigating unexpected adverse drug reactions, solidifying its dominance in the pharmacovigilance landscape.
- Contract Outsourcing: Emerging as the fastest-growing service provider, contract outsourcing gains traction due to its cost-effectiveness, flexibility, and efficient resource sharing.
- Oncology Market Growth: The increasing emphasis on monitoring cancer drug safety, particularly in addressing side effects, stands out as a key driver fueling growth within the pharmacovigilance industry.
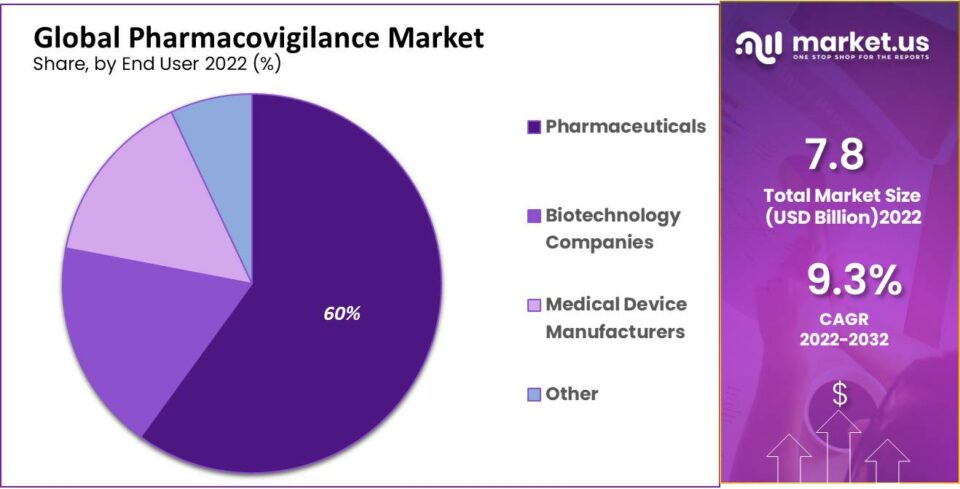
- Pharmaceutical Companies: Maintaining a substantial market share, pharmaceutical companies are at the forefront, driven by the escalating adoption of pharmacovigilance services to ensure drug safety and regulatory compliance.
Factors affecting the growth of the Pharmacovigilance industry
- Regulatory Landscape: The regulatory environment profoundly impacts the Pharmacovigilance sector. Stringent regulations and evolving compliance standards necessitate pharmaceutical companies to invest in robust pharmacovigilance systems. Adherence to these regulations not only ensures patient safety but also propels the industry forward.
- Globalization of Clinical Trials: With an increasing trend towards global clinical trials, the Pharmacovigilance industry is expanding its reach. Conducting trials in diverse geographical locations necessitates a comprehensive approach to monitoring drug safety worldwide. This globalization trend fuels the demand for pharmacovigilance services to ensure consistent and thorough surveillance across different regions.
- Rising Drug Complexity and Diversity: The pharmaceutical landscape is witnessing a surge in the complexity and diversity of drugs. As the industry develops more specialized and targeted therapies, the need for vigilant monitoring of potential adverse effects grows. Pharmacovigilance becomes instrumental in managing the intricate safety profiles of these advanced drugs, contributing significantly to the industry's growth.
Regional Analysis
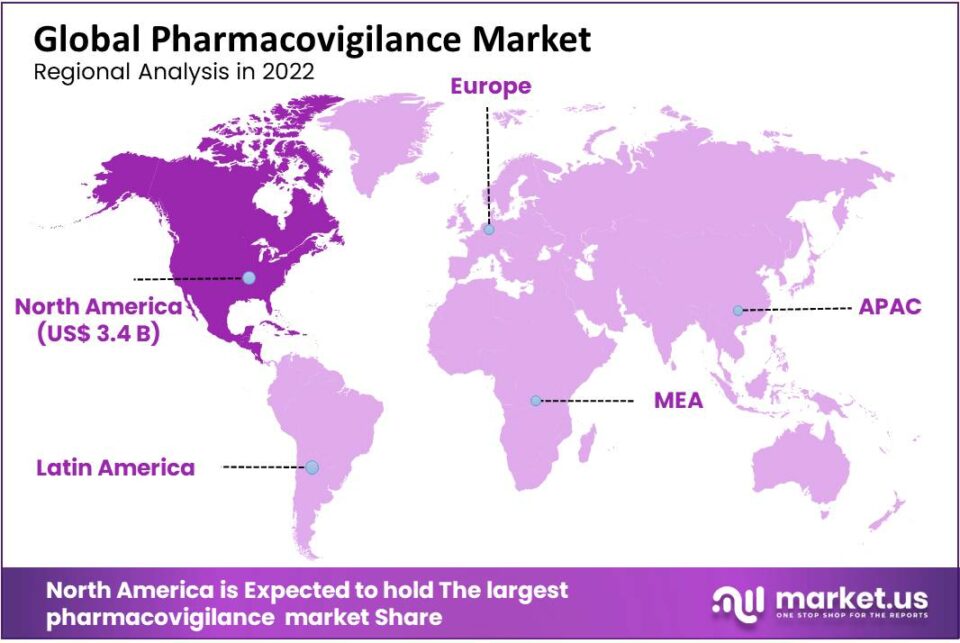
North America commands a significant share in the pharmacovigilance market, primarily driven by the presence of key players in the pharmaceutical and medical device sectors. The region's dominance in revenue is attributed to these industries' substantial contributions. Notably, the escalating issues of drug abuse and related adverse reactions, causing considerable morbidity and mortality, act as a catalyst for market growth. The North American market benefits from this as a major growth factor.
Furthermore, the market expansion is propelled by substantial investments from key players in innovative drug development. This has led to a surge in clinical trials and an increased need for post-marketing surveillance, aligning with the region's extensive drug production and fostering overall expansion.
To understand how our report can make a difference to your business strategy, Inquire about a brochure at https://market.us/report/pharmacovigilance-market/#inquiry
Scope of the Report
Scope of the Report
| Report Attributes | Details |
| Market Value (2023) | US$ 8.5 Billion |
| Forecast Revenue 2032 | US$ 18.6 Billion |
| CAGR (2023 to 2032) | 9.3% |
| North America Value | USD 3.4 Bn in 2023 |
| Base Year | 2022 |
| Historic Period | 2018 to 2022 |
| Forecast Year | 2024 to 2033 |
Market Drivers
The industry experiences significant momentum propelled by the escalating prevalence of chronic diseases. This surge in chronic conditions, such as cancer survivors grappling with persistent pain, fuels a heightened demand for pain relievers, intensifying the overall market. Moreover, conditions like diabetes, cardiovascular disease, and autoimmune disorders necessitate drug combinations, potentially leading to adverse reactions, prompting the application of pharmacovigilance. Notably, government-established stringent regulations and the continual emergence of novel pharmaceuticals further propel industry advancement. Regulatory standards for clinical studies, set by entities like the European Medicines Agency and the US Food and Drug Administration, contribute to this progress.
Additionally, the pharmacovigilance sector expands through government initiatives, charitable organizations, and increased awareness campaigns on drug safety. The industry's growth is further propelled by the global COVID-19 pandemic, heightening the demand for effective medications to combat the virus.
Market Restraints
The growth of the pharmacovigilance market is hindered in certain countries due to a lack of awareness and regulatory frameworks. Insufficient resources and budget constraints pose challenges for some companies in conducting effective pharmacovigilance. Additionally, technical limitations, data accuracy concerns, and privacy issues contribute to the market's sluggish growth. The absence of standardized practices further exacerbates these issues. Implementation costs are high, further impeding progress. To overcome these obstacles, there is a crucial need for increased awareness, regulatory alignment, and strategic resource allocation to fortify pharmacovigilance efforts globally.
Market Opportunities
Pharmacovigilance stands as a systematic advancement within the pharmaceutical sector, an intrinsic element in drug development and manufacturing. In this indispensable process, pharmacovigilance pharmacists play a vital role, in ensuring the safe and appropriate utilization of medications. Their primary task involves the identification of risk factors associated with adverse drug reactions (ADRs). This meticulous approach not only safeguards users but also promotes the exploration of previously undiscovered ADRs and their potential interactions with known adverse reactions. In essence, pharmacovigilance serves as a proactive mechanism, contributing to the ongoing enhancement of drug safety and efficacy in the ever-evolving landscape of pharmaceuticals.
Immediate Delivery Available | Buy This Premium Research Report https://market.us/purchase-report/?report_id=96646
Report Segmentation of the Pharmacovigilance Market
Type Insight
The dominant revenue share in pharmacovigilance is held by spontaneous reporting, owing to its widespread application and the ability to simulate data sets for effective drug comparison. This method is crucial for detecting new, serious, and uncommon adverse drug reactions (ADRs), offering a cost-effective strategy. The market's significant growth is attributed to the extensive utilization of surveillance reports by pharmaceutical companies and regulatory authorities. Cohort event monitoring (CEM) emerges as the second-largest segment, gaining popularity due to its active surveillance approach using statistical tools and data mining systems, particularly with longitudinal health records.
The rising prominence of CEM is driven by its effectiveness in detecting adverse clinical events for both new and existing medicines. The European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) predicts targeted spontaneous reporting as the fastest-growing segment, spurred by government initiatives diversifying reporting methods. This anticipates lower costs, enhanced affordability, and routine monitoring advantages. The utilization of electronic health records (EHR) further contributes to market expansion, with benefits including improved clinical workflow, patient management, automated access, enhanced quality of care, and better clinical decision-making, particularly post-hospital discharge.
Product Life Cycle Insight
The dominance of Phase IV in the product life cycle is attributed to extensive post-marketing surveillance of pharmaceuticals and a rise in adverse drug reaction incidents. This segment holds a significant share in the pharmacovigilance market, offering an added layer of protection during clinical trials. Detecting unforeseen drug reactions is crucial at this stage, making Phase IV indispensable. The substantial data collected from extensive testing on a diverse patient base becomes pivotal post-commercialization. Forecasts predict a profitable 10.5% growth in the Phase III segment during the forecast period. Phase III trials not only establish drug efficacy but also provide vital information on drug interactions, safety, and effectiveness, contributing to increased revenue generation in the sector.
Process Flow Insight
Healthcare professionals primarily use Spontaneous Reporting Systems (SRSs) to voluntarily notify regulatory bodies of suspected cases, fostering accurate signal detection. This entails leveraging database knowledge, high-quality data, and tools for data summary, visualization, and evaluation. Currently, businesses harness big data and artificial intelligence (AI) to enhance signal assessment. The case data management segment is expected to experience rapid growth over the forecast period. Adverse event information is generated through post-marketing programs, clinical trials, spontaneous reports, and literature. This multifaceted approach ensures comprehensive monitoring and reporting within the healthcare system, supporting the continuous improvement of patient safety.
Therapeutic Area Insight
Monitoring the safety of cancer drugs is crucial due to potential side effects, driving demand for pharmacovigilance services. Both targeted and conventional anti-cancer medications carry risks, emphasizing the need for prevention, detection, and management of adverse effects. The oncology market is anticipated to grow rapidly, spurred by the intrinsic toxicity and limited therapeutic window of these drugs. Accelerated research and development respond to the increasing cancer incidence. Pharmacovigilance facilitates early detection and reporting of adverse reactions, vital for patient safety. Recent advances like targeted therapy pose challenges to patients' quality of life. Monoclonal antibodies, a significant category, generated numerous spontaneous adverse reaction reports. Serious adverse drug reactions have become a major health concern, contributing to over 100,000 annual deaths in the US alone, fueled by evolving reporting systems and technology.
Service Provider Insights
In the pharmaceutical sector, there's a notable shift towards outsourcing services, primarily driven by the desire to trim operational costs. Contract outsourcing holds a significant market share and is expected to experience the fastest expansion in the forecast period. The appeal lies in its advantages lower fixed costs, reduced upfront investments, lower risk, and increased resource flexibility. Contract outsourcing companies offer tailored services like PV audits and process design Standard Operating Procedures (SOP). The rise of Clinical Research Organizations (CROs) in Asia Pacific, particularly in India, China, and Japan, contributes to dynamic growth. Outsourcing PV services brings benefits like meeting cost targets, scalability for expanding portfolios, and navigating complex regulatory requirements. Meanwhile, in-house markets are projected to see moderate growth due to extensive R&D efforts by major pharmaceutical and biotech companies in developing new drugs. This signifies potential industry growth in the coming years.
End-User Insight
The hospital sector dominates the market share, propelled by the surge in ADR-related hospital admissions. Research organizations are poised for rapid expansion, fueled by increased funding for pharmaceutical efficiency. The pharmaceutical industry thrives on novel treatments, holding the largest market share. To bolster capacity and cut costs, pharma companies outsource pharmacovigilance. This proves especially cost-effective for smaller businesses. The biotechnology sector is expected to witness lucrative growth due to heightened activities in new product development within pharmacovigilance. With a rising drug consumption rate, there's a growing concern for undiscovered side effects over prolonged use. Regulatory authorities' increasing demand for medical information further propels this segment.
Stay informed about market trends and growth opportunities: https://market.us/report/pharmacovigilance-market/request-sample/
Market Segmentation
By Service Provider
- In-house
- Contract Outsourcing
- Others
By Product Life Cycle
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Type
- Spontaneous Monitoring
- Intensified ADR Monitoring
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- Others
By Process Flow
- Case Data Management
- Signal Detection
- Risk Management System
- Others
By Therapeutic Area
- Oncology
- Neurology
- Cardiology
- Pulmonology
- Others
Based By End-User
- Pharmaceuticals
- Biotechnology Companies
- Medical Device Manufacturers
- Others
By Geography
- North America
- US
- Canada
- Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Competitive Landscape
The pharmaceutical landscape is witnessing a remarkable expansion due to a surge in new drug developments and the expiration of brand-name drug patents. This growth has led to a fragmented global market with numerous large and medium-sized players dominating revenue shares. Major industry participants are strategically leveraging mergers, acquisitions, and partnerships to advance research and development, introducing more effective therapeutic drugs. The competitive environment has attracted pharmacovigilance service providers globally, enhancing research efficiency and clinical data management. Ongoing collaborations and alliances among key players, such as Accenture and UCB, underscore the commitment to accelerating data processing and improving patient safety, reflecting the dynamic and competitive nature of the pharmacovigilance market.
Market Key Players
- Accenture plc
- Bristol-Myers Squibb Company
- Clinquest Group B.V.
- Cognizant Technology Solutions Corporation
- GlaxoSmithKline plc
- ICON plc
- Novartis AG
- Hoffmann-La Roche Ltd.
- PAREXEL International Corporation
- Pfizer Inc.
- ICON plc
- Wipro Limited
Recent Developments in the Pharmacovigilance Market
- February 2023: Cognizant partnered with Medable Inc. to offer combined clinical research solutions focused on decentralized clinical trials, impacting pharmacovigilance data collection.
- April 2023: ICON acquired the clinical research business of PRA Health Sciences, enhancing its global footprint and expertise in data collection and management, impacting pharmacovigilance data acquisition.
- May 2023: Accenture partnered with Oracle Health Sciences to integrate Oracle's Argus Safety platform with Accenture's myHealth platform for a more holistic patient safety solution.
- June 2023: Clinquest Group acquired the pharmacovigilance business of PAREXEL International Corporation, expanding its global reach and service offerings.
- July 2023: Bristol-Myers Squibb partnered with Aetion, a real-world evidence company, to develop new methods for identifying and assessing drug safety risks.
- September 2023: GlaxoSmithKline announced the expansion of its global pharmacovigilance center in India, strengthening its data analysis capabilities and creating new jobs.
Browse More Related Reports
- Life Sciences BPO Market size is expected to be worth around USD 999 Billion by 2033, from USD 406.8 Billion in 2023, growing at a CAGR of 9.4%.
- Life Science Analytics Market was valued at USD 9 billion in 2022, and expected to grow around USD 19 billion in 2032 at a CAGR of 8%.
- Specialty Pharmaceutical Market size is expected to be worth around USD 1532.8 billion by 2033, from USD 68.3 billion in 2023, growing at a CAGR of 36.5%.
- ePharmacy Market size is anticipated to reach approximately USD 258.6 billion by the year 2033, exhibiting substantial growth from USD 72.9 billion in 2023, at a CAGR of 19.8%.
- Pharmaceutical Filtration Market size is expected to be worth around USD 28.7 billion by 2032, from USD 12.8 billion in 2023, growing at a CAGR of 9.4%.
- Active Pharmaceutical Ingredient Market size is expected to be worth around USD 839.1 Billion by 2033, from USD 236.5 Billion in 2023, growing at a CAGR of 6.2%.
- Generic Pharmaceuticals Market was worth USD 346.5 Billion in 2022. It is expected to reach USD 740.5 Billion by 2032, growing at a CAGR of 8.1%.
- Pharmaceutical Cartridges Market size is expected to be worth around USD 3.4 Billion by 2032 from USD 1.7 Billion in 2022, growing at a CAGR of 7.5%.
About Us:
Market.US (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. Market.US provides customization to suit any specific or unique requirement and tailor-makes reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us on LinkedIn | Facebook | Twitter
Our Blog:
