Dublin, Jan. 08, 2024 (GLOBE NEWSWIRE) -- The "Global Cell and Gene Therapy CDMO Market: Analysis By Type, Application, Indication, By Region, By Country: Market Insights and Forecast" report has been added to ResearchAndMarkets.com's offering.
The Global Cell and Gene Therapy CDMO market valued at USD 2.54 billion in 2022 is expected to grow at a CAGR of 30.75% during the forecast period of 2024-2029.
The report provides a complete analysis of the Global Cell and Gene Therapy CDMO Market industry in terms of historical data for 2019-2022, estimates of 2023 and the forecasts for 2024-2029. 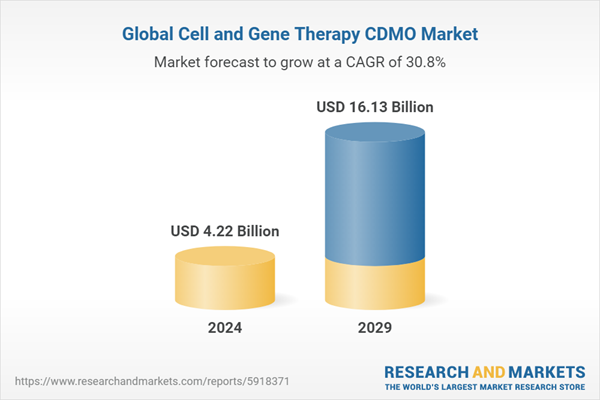
The global Cell and Gene Therapy CDMO market has been experiencing robust growth and escalating demand, propelled by continuous advancements in cell and gene therapies. With a broadening scope of therapeutic areas and a favourable regulatory environment, the global Cell and Gene Therapy CDMO market is poised for sustained expansion as it plays a pivotal role in the development and commercialization of ground-breaking therapies for various diseases. Global Cell and Gene Therapy CDMO market is expected to grow at an extraordinary rate driven by significant investments from industry players.
The global pipeline of cell and gene therapies has been expanding, covering a wide range of therapeutic areas, including oncology, rare diseases, and genetic disorders. As more therapies advance through clinical trials and toward commercialization, the demand for CDMO services for manufacturing, process development, and scale-up increases.
Global CDMO organisations for cell and gene therapy are expanding, and this is one of the main factors propelling the global Cell and Gene Therapy's overall expansion. With the increasing global demand for advanced cell and gene therapies, CDMOs are proactively setting up production facilities in various locations to meet the changing demands of biotechnology and pharmaceutical companies. These days, CDMOs are essential to the manufacturing of pharmaceuticals.
Over the past ten years, CDMOs have grown in prominence due to a more dynamic mergers and acquisition landscape. Serving both local and international markets, there are currently over 600 active Contract Development and Manufacturing Organizations (CDMOs). CDMOs provide specific expertise that helps both "Big Pharma"and smaller inventors to launch products sooner.
The rise of biologics is seen by the number of CDMOs making significant investments in brand-new biomanufacturing facilities. These companies include Boehringer Ingelheim (USD827M investment), Samsung Biologics (USD1.7B investment), Lonza (USD935M expansion), and Fujifilm Diosynth Biotechnologies (USD2B investment).
Additionally, by expanding globally, CDMOs can meet regional regulatory needs, provide customised manufacturing solutions, and improve accessibility for customers that need their services. CDMOs can provide more adaptable and responsive solutions by being present in important worldwide markets. This helps to speed up the development and global commercialization of gene and cell therapies.
One prominent factor restraining the growth of the global Cell and Gene Therapy CDMO market is the high cost for producing viral vectors. As carriers of therapeutic genes into target cells, viral vectors are essential to the development of gene and cell therapies. Viral vector production is a complex procedure that frequently involves specialized techniques, stringent quality control standards, and specialized facilities.
There are now five viral vectors in use for gene therapy. AAV have the biggest market share, followed by lentiviruses, adenoviruses, herpes simplex viruses and retroviruses. The vector manufacturing cost for a single dose might be as high as USD 1-2 million, given the expected price of USD 3-7 million per batch, according to Roland Berger Insights. Therefore, the high cost of producing viral vectors hampers cell and gene therapies from being widely commercialised and made accessible, which limits their uptake and hinders the expansion of the Cell and Gene CDMO market.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 260 |
| Forecast Period | 2024 - 2029 |
| Estimated Market Value (USD) in 2024 | $4.22 Billion |
| Forecasted Market Value (USD) by 2029 | $16.13 Billion |
| Compound Annual Growth Rate | 30.7% |
| Regions Covered | Global |
Scope of the Report
- The report analyses the Global Cell and Gene Therapy CDMO Market by Value (USD Million).
- The report presents the analysis of Global Cell and Gene Therapy CDMO Market for the historical period of 2019-2022, the estimated year 2023 and the forecast period of 2024-2029.
- The report analyses the Global Cell and Gene Therapy CDMO Market by Type (Cell Therapy, Gene Therapy, Gene-modified Cell Therapies).
- The report analyses the Cell and Gene Therapy CDMO Market by Application (Clinical, Pre-clinical).
- The report analyses the Cell and Gene Therapy CDMO Market by Indication (Oncology Diseases, Infectious Diseases, Neurological Disorder, Other Indications).
- The report analyses the Cell and Gene Therapy CDMO Market by Region (Americas, Europe, Asia Pacific, Middle East & Africa).
- The report analyses the Cell and Gene Therapy CDMO Market by Country (United States, Canada, United Kingdom, Germany, France, Italy, China, Japan, South Korea, India).
- The key insights of the report have been presented through the frameworks of SWOT and Porter's Five Forces Analysis.
- Also, the major opportunities, trends, drivers and challenges of the industry has been analyzed in the report.
- The report tracks competitive developments, strategies, mergers and acquisitions and new product development.
Competitive Positioning
- Companies' Product Positioning
- Competitive Positioning of Oxford Biomedica's Viral Vector Offering
- Market Position Matrix
- Market Share Analysis of Cell and Gene Therapy CDMO Market
Company Profiles
- Oxford Biomedica
- Lonza Group AG
- Catalent, Inc.
- Samsung Biologics
- WuXi Biologics
- Pfizer CentreOne
- Danaher Corporation
- Thermo Fisher Scientific Inc.
- Charles River Laboratories International, Inc.
- Novartis AG
For more information about this report visit https://www.researchandmarkets.com/r/rlsb3z
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
