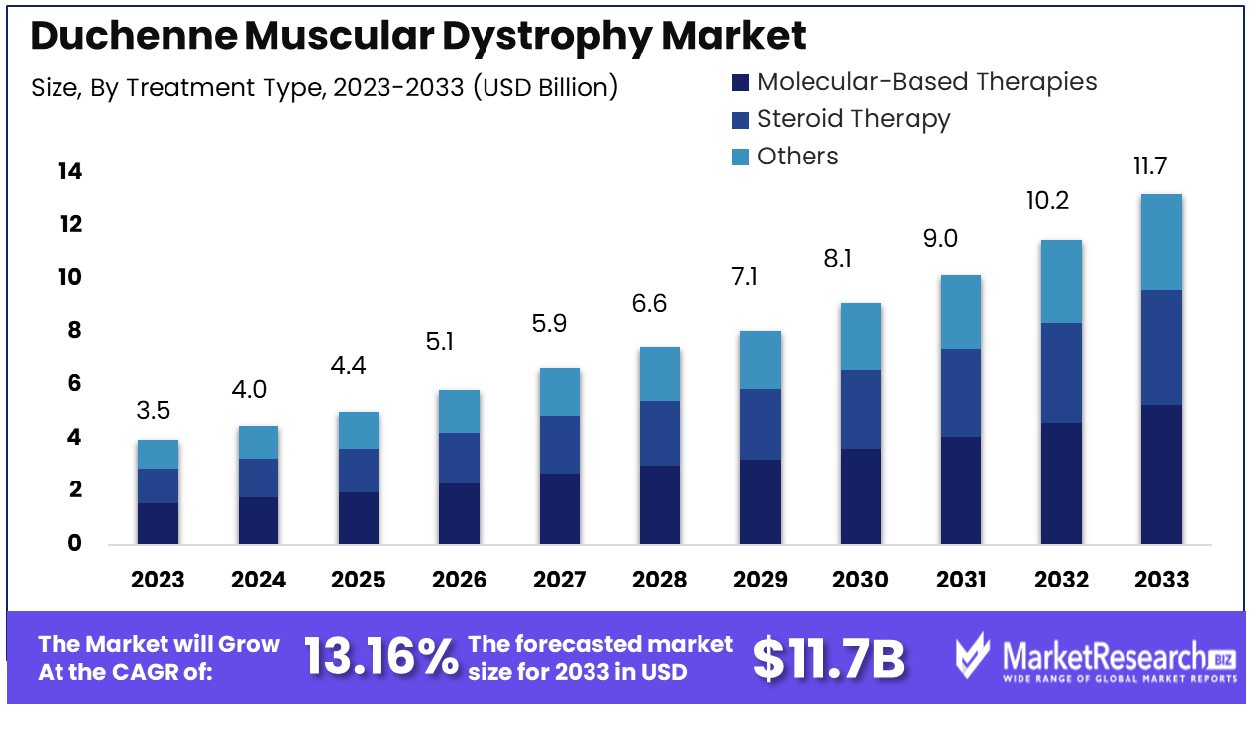
New York, Jan. 24, 2024 (GLOBE NEWSWIRE) -- Duchenne muscular dystrophy market was valued at USD 3.5 billion in 2023 with significant growth and is projected to reach USD 11.7 billion by 2033 with an outstanding CAGR of 13.16%.
The Duchenne Muscular Dystrophy (DMD) market is propelled by a surge in demand for advanced diagnostics and the associated high cost of therapeutics. DMD, a severe form of dystrophin-related muscular dystrophy, manifests in childhood, with initial symptoms emerging before the age of 4, leading to muscle weakness and dependency on wheelchairs by age 12. Respiratory difficulties and heart failures contribute to mortality by the third decade. Global prevalence, highlighted in reports, underscores the impact, with America having the highest occurrence at 5.1 per 100,000 individuals.
Get additional highlights on major revenue-generating segments, Request a Duchenne Muscular Dystrophy Market sample report at https://marketresearch.biz/report/duchenne-muscular-dystrophy-market/request-sample/
DMD, primarily hereditary, affects 1 in 3,600 male live-born babies, with 30% of cases due to spontaneous genetic mutations. Carriers, particularly females, may exhibit milder signs. While DMD currently lacks a cure, supportive therapies include physical therapy, surgeries for contractures, mobility aids, and tracheostomy. Ongoing clinical trials for drugs like Exon skipping, targeting mutated dystrophin genes, offer promise and have FDA approval.
The rising incidence of DMD and ongoing research signal a growing market, driven by the imperative need for advanced diagnostics and therapeutics to address this debilitating genetic disorder. The forecasted expansion reflects a commitment to improving the quality of life for those affected by DMD in the years to come.
Key Takeaways
- Molecular-based therapies dominate the treatment type market segment due to innovative treatments that aim to modify or correct the dystrophin gene mutations responsible for DMD.
- Exon skipping approach leads the therapy market segment due to a significant portion of DMD patients, depending on the specific exon mutations they carry.
- Oral care administration rules the route of administration market segment due to its preference for its ease and non-invasiveness, especially for long-term treatments.
- Hospitals dominate the end-user market segment due to their multidisciplinary care, making hospitals a central location for comprehensive treatment.
- Hospital pharmacies hold a strong position in the distribution channel market segment due to its better equipped to handle the distribution and administration of these treatments.
- North America commands a 40% share in the Duchenne Muscular Dystrophy (DMD) market, driven by advanced healthcare, robust research, and heightened rare disease awareness.
Driving Factors
Enhanced Diagnosis Pushes Duchenne Muscular Dystrophy Market
The Duchenne muscular dystrophy market is witnessing growth factors due to advancements in genetic testing and screening. Earlier, it took families an average of 2.2 to 2.3 years to receive a DMD diagnosis. Now, enhanced diagnostic techniques, like the identification of a unique epigenetic signature, are allowing for earlier and more accurate diagnosis in infants and children. This development not only expands the patient pool but also opens avenues for early intervention, potentially altering the disease's progression and treatment approach.
Augment Disease Awareness Diagnosis and Treatment
Patient advocacy and support groups, such as the Parent Project Muscular Dystrophy and the Muscular Dystrophy Association (MDA), play a crucial role in raising awareness about DMD. Their efforts contribute to earlier diagnosis and improved access to care. Initiatives like National Muscular Dystrophy Awareness Month amplify the understanding of DMD, fostering community support and encouraging research. This heightened awareness is instrumental in driving market growth by ensuring that patients receive timely and effective care.
To understand how our report can bring a difference to your business strategy, Inquire about a brochure at https://marketresearch.biz/report/duchenne-muscular-dystrophy-market/#inquiry
Restraining Factors
High-priced Treatment Costs Restrict Accessibility
The high cost of treatment significantly limits the growth of the Duchenne Muscular Dystrophy (DMD) market. Traditional DMD therapies can accumulate direct costs as high as $2.3 million over a 20-year treatment period. Newer gene and molecular therapies are even more costly, reaching hundreds of thousands of dollars per patient annually. These exorbitant costs pose a substantial barrier to treatment adoption and make reimbursement a challenging process, significantly hindering market growth by restricting patient access due to financial constraints.
Growth Opportunities
Advanced Pain Management Solutions
Pain management is a critical aspect of improving the quality of life for DMD patients, who often suffer from severe nerve, muscle, and joint pain. Developing better pain relief solutions tailored to the unique needs of DMD patients can significantly enhance their daily living. This not only meets a vital patient need but also opens up a market for innovative therapies and medications specifically designed for pain management in DMD, potentially leading to increased investment and research in this area.
Have Queries? Speak to an expert or To Download/Request a Sample, Click here.
| Report Attribute | Details |
| Market Value (2023) | US$ 3.5 Billion |
| Market Size (2033) | US$ 11.7 Billion |
| CAGR (from 2024 to 2033) | 13.16% from 2024 to 2033 |
| North America Region Revenue Share | 40% |
| Historic Period | 2016 to 2023 |
| Base Year | 2023 |
| Forecast Year | 2024 to 2033 |
Regional Analysis
North America commands a 40% share in the Duchenne Muscular Dystrophy (DMD) market, driven by advanced healthcare, robust research, and heightened rare disease awareness. The United States, led by Sarepta Therapeutics and Pfizer, dominates DMD research. FDA initiatives, collaboration, and supportive policies fuel market dynamics. In Europe, a focus on research and regulatory support, especially from EMA, propels the market. Asia-Pacific's emerging market sees growth with increased healthcare investment, awareness, and research, offering substantial potential for DMD market expansion.
Segment Analysis
By treatment type analysis, molecular-based therapies dominate the market segment due to innovative treatments that aim to modify or correct the dystrophin gene mutations responsible for DMD. This segment's leadership stems from the targeted approach these therapies take to address the underlying genetic cause of DMD. Other treatment types include supportive care and physiotherapy but are secondary to molecular-based therapies in terms of disease-modifying potential.
By therapy analysis, exon skipping approach leads the market segment due to a significant portion of DMD patients, depending on the specific exon mutations they carry. This method involves the use of antisense oligonucleotides to skip over faulty parts of the dystrophin gene, allowing for the production of a partially functional dystrophin protein. Mutation suppression involves therapies that enable the cellular machinery to read through a mutation, while Dystrophin-Targeted Therapies focus on replacing or repairing the dysfunctional dystrophin protein.
By route of administration analysis, oral care administration rules the market segment due to its preference for ease and non-invasiveness, especially for long-term treatments. Parenteral routes, which include intravenous and subcutaneous injections, are used for therapies that cannot be effectively delivered orally or require targeted delivery.
By end-user analysis, hospitals dominate the market segment due to their multidisciplinary care, making hospitals a central location for comprehensive treatment. These Hospitals are equipped with the necessary infrastructure for advanced therapies and can provide integrated care involving specialists, physiotherapists, and other healthcare professionals.
By distribution channel analysis, hospital pharmacies hold a strong position in the market segment due to its better equipped to handle the distribution and administration of these treatments, especially those that require close medical supervision or are administered via parenteral routes. The need for professional oversight of many DMD treatments reinforces the dominance of Hospital Pharmacies in this market.
For more insights on the historical and Forecast market data from 2016 to 2033 - download a sample report at https://marketresearch.biz/report/duchenne-muscular-dystrophy-market/request-sample/
Segment covered in this report
By Treatment Type
- Molecular-Based Therapies
- Steroid Therapy
- Others
By Therapy
- Exon Skipping Approach
- Mutation Suppression
- Dystrophin-Targeted Therapies
By Route of Administration
- Oral
- Parenteral
- Others
By End User
- Hospitals
- Home Healthcare
- Specialty Clinics
- Others
By Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Grow your profit margin with Marketresearch.biz - Purchase This Premium Report at https://marketresearch.biz/purchase-report/?report_id=43013
Competitive Landscape Analysis
In the Duchenne Muscular Dystrophy (DMD) Market, Sarepta Therapeutics and Pfizer Inc. lead in driving innovation and drug development, emphasizing advanced gene therapy. PTC Therapeutics and F. Hoffmann-La Roche AG contribute significantly to DMD treatment, focusing on therapies to slow disease progression. Their commitment to extensive research and clinical trials underscores the market's dedication to developing effective, long-term treatments, shaping the landscape of DMD therapeutic advancements.
Key Players
- Pfizer Inc.
- Sarepta Therapeutics
- PTC Therapeutics
- FibroGen Inc.
- F. Hoffmann-La Roche AG
- Nobelpharma Co. Ltd
- NS Pharma Inc.
- Santhera Pharmaceuticals
- ReveraGen BioPharma
- Lexicon Pharmaceuticals. Inc.
Recent Developments
- In Jan 2024, The early findings from therapy designated DYNE-251 weighed down on rival Sarepta Therapeutics, which makes several drugs to treat the condition, pushing its shares down 2.1% to $94.19 after the markets opened. Stifel analyst Paul Matteis said in a note that DMD data shows a promising start, as the dose is smaller and efficacy is better than Sarepta’s drug.
- In Nov 2023, Solid Biosciences Announces IND Clearance by FDA for Duchenne Muscular Dystrophy Gene Therapy Candidate SGT-003.
- In October 2023, Santhera developed vamorolone in collaboration with ReveraGen BioPharma. The drug is currently an approved decision by the US Food and Drug Administration (FDA) Prescription Drug User Fee Act (PDUFA). Vamorolone has an Orphan Drug status in the US and EU, along with a Promising Innovative Medicine (PIM) status in the UK for treating DMD.
Browse More Related Reports
- Physical Therapy Market is projected to reach USD 49.0 Bn by 2032 from USD 27.0 Bn in 2022, with a CAGR of 6.3% from 2023 to 2032.
- Orthopedic Devices Market is anticipated to be valued at USD 74.9 Bn by 2032, up from USD 54.1 Bn in 2022, exhibiting a CAGR of 3.4% during 2023-2032.
- Collagen Supplements Market is set to grow to USD 74.7 Bn by 2032, compared to USD 1.8 Bn in 2022, reflecting a CAGR of 6.6% from 2023 to 2032.
- Orthopedic Regenerative Surgical Products Market is forecasted to achieve a worth of USD 8.6 Billion by 2032, increasing from USD 5.2 Billion in 2022, with a CAGR of 5.3% during 2023-2032.
- Physiotherapy Equipment Market is poised to reach USD 37.5 Bn by 2032, rising from USD 22.1 Bn in 2022, demonstrating a CAGR of 5.6% from 2023 to 2032.
About Us:
MarketResearch.Biz (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. MarketResearch.Biz provides customization to suit any specific or unique requirement and tailor-made reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us on LinkedIn: https://www.linkedin.com/company/marketresearch-biz/
Follow Us on Facebook: https://www.facebook.com/marketresearch.biz
Follow Us on Twitter: https://twitter.com/PrudourResearch
