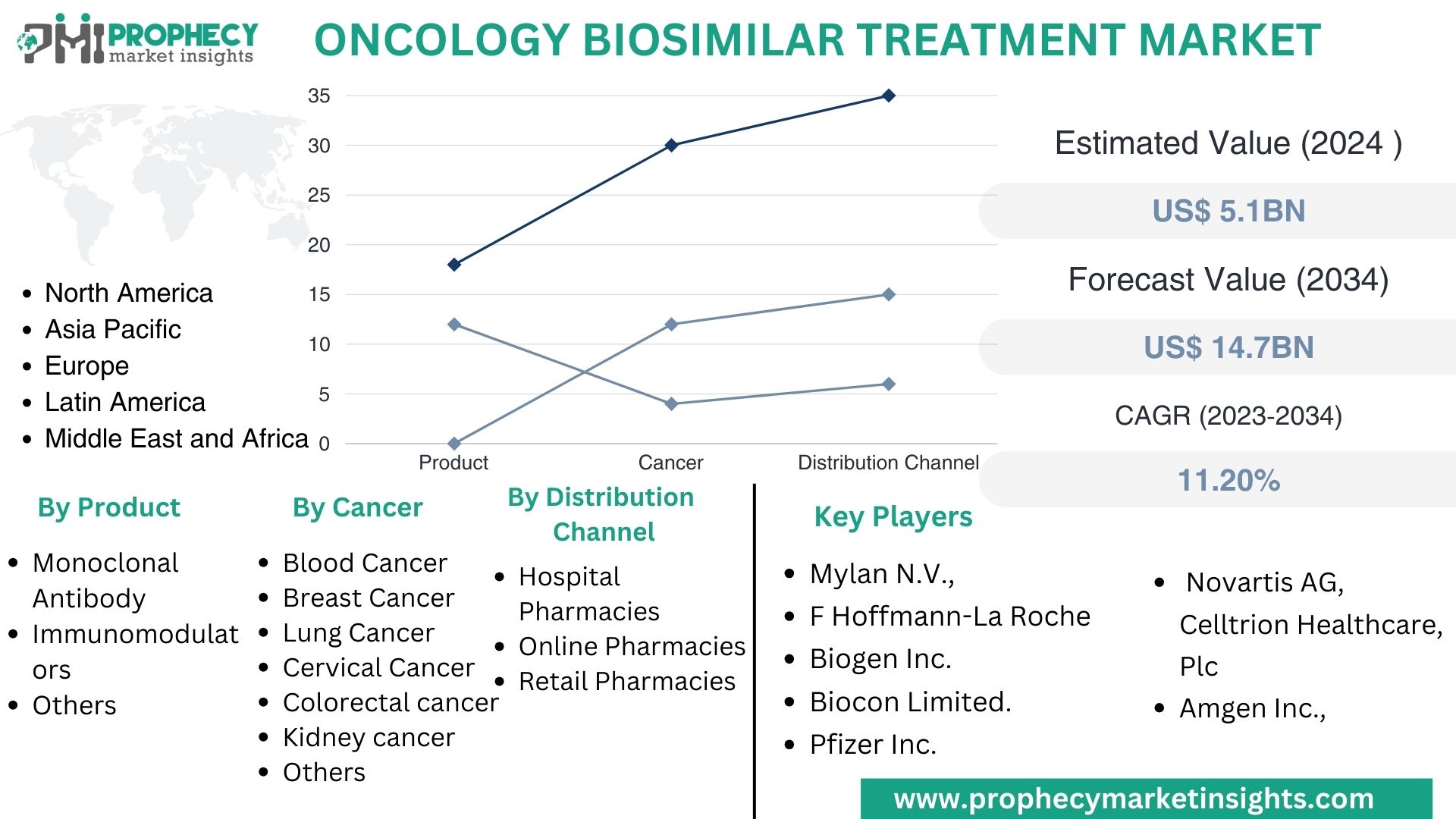
Covina, Feb. 07, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the Oncology Biosimilar Treatment Market size was valued at about USD 5.1 Billion in 2024 and expected to grow at CAGR of 11.20% to extend a value of USD 14.7 Billion by 2034.”
What is Oncology Biosimilar Treatment?
- Market Overview:
Oncology biosimilar treatment refers to the use of biosimilar medications in the treatment of cancer. Biosimilars are biologic drugs that are highly similar to existing biologic medications, known as reference or originator drugs. They are developed to have comparable efficacy, safety, and quality to the original biologic drug.
In oncology, biosimilars are used to treat various types of cancer, including but not limited to breast cancer, colorectal cancer, lung cancer, and leukemia. These biosimilar treatments may include monoclonal antibodies, growth factors, and other targeted therapies that play a crucial role in cancer management and treatment.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/1096
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Top Leading Players in Oncology Biosimilar Treatment Market:
- Mylan N.V.
- F Hoffmann-La Roche
- Biogen Inc.
- Biocon Limited.
- Novartis AG
- Celltrion Healthcare, Plc.
- Amgen Inc.
- Pfizer Inc.
- Allergan
- Teva Pharmaceutical Industries Ltd.
Market Dynamics:
Driving Factors:
- Biosimilar treatments typically offer cost savings compared to their reference biologics. This cost-effectiveness drives their adoption, particularly in oncology, where treatment expenses can be substantial.
- The rising incidence of cancer worldwide necessitates more affordable treatment options. Oncology biosimilars offer a cost-effective alternative to expensive biologics, contributing to their growing demand.
- As patents for originator biologics expire, it creates opportunities for biosimilar manufacturers to enter the market. This increases competition and expands the availability of biosimilar treatments in oncology.
- Regulatory agencies have established pathways to expedite the approval process for biosimilars. This supportive regulatory environment encourages the development and commercialization of oncology biosimilars.
- Healthcare professionals and patients are becoming more familiar with biosimilars, leading to increased acceptance and adoption. This growing awareness contributes to the expansion of the oncology biosimilar treatment market.
Restrain Factors:
- Complex Development Process
- Concerns Regarding Safety and Efficacy
- Market Access Barriers
- Legal and Intellectual Property Issues
- Lack of Interchangeability
Emerging Trends and Opportunities in Oncology Biosimilar Treatment Market:
- The pipeline of oncology biosimilars continues to grow, with an increasing number of biologics facing patent expiry. This expansion presents opportunities for biosimilar developers to enter the market and offer more affordable treatment options across various cancer types.
- Monoclonal antibodies play a crucial role in cancer treatment, and biosimilar versions of popular monoclonal antibodies such as trastuzumab and rituximab are gaining traction. Developers are focusing on expanding the portfolio of monoclonal antibody biosimilars for oncology indications, offering patients more options for targeted therapy.
- Combining biosimilars with other cancer treatments, including chemotherapy and targeted therapies, is an emerging trend in oncology. Biosimilar combinations have the potential to enhance treatment efficacy, reduce adverse effects, and improve patient outcomes.
- Regulatory agencies are increasingly granting extrapolation of indications for biosimilars based on comprehensive analytical and clinical data. This allows biosimilar developers to obtain approval for multiple indications, expanding the market potential of oncology biosimilars.
- Education and awareness initiatives targeting healthcare professionals, patients, and policymakers play a crucial role in driving biosimilar adoption in oncology. Efforts to increase understanding of biosimilars, including their safety, efficacy, and regulatory pathways, can facilitate market penetration and acceptance.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/1096
Challenges of Oncology Biosimilar Treatment Market:
- Regulatory approval processes for biosimilars are stringent and complex, requiring extensive analytical and clinical data to demonstrate similarity to reference biologics. Meeting regulatory requirements poses a significant challenge for biosimilar developers, leading to delays and uncertainties in market entry.
- Despite regulatory approval, concerns regarding the safety and efficacy of biosimilars compared to reference biologics may persist among healthcare professionals and patients. Lack of confidence in biosimilar products can hinder their adoption in clinical practice, especially in oncology where treatment decisions have critical implications for patient outcomes.
- Biologic drugs, including biosimilars, face market access barriers such as formulary restrictions, reimbursement limitations, and payer preferences. Negotiating favorable formulary placement and reimbursement terms presents a significant challenge for biosimilar manufacturers, affecting product uptake and market penetration.
- The oncology biosimilar market is characterized by intense competition among manufacturers, leading to pricing pressures and margin erosion. Differentiating biosimilar products based on clinical attributes, patient outcomes, and value propositions is essential to gain market share and sustain competitive advantage.
Detailed Segmentation:
Oncology Biosimilar Treatment Market, By Product:
-
-
- Monoclonal Antibody
- Immunomodulators
- Others
-
Oncology Biosimilar Treatment Market, By Cancer:
-
-
- Blood Cancer
- Breast Cancer
- Lung Cancer
- Cervical Cancer
- Colorectal cancer
- Kidney cancer
- Others
-
Oncology Biosimilar Treatment Market, By Distribution Channel:
-
-
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
-
Oncology Biosimilar Treatment Market, By Region:
-
-
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
-
- Asia Pacific
-
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Mexico
- Rest of Latin America
-
- Middle East & Africa
-
- GCC
- Israel
- South Africa
- Rest of Middle East & Africa
-
- North America
-
Regional Analysis:
Regional insights highlight the diverse market dynamics, regulatory landscapes, and growth drivers shaping the Oncology Biosimilar Treatment Market across different geographic areas. Understanding regional nuances and market trends is essential for stakeholders to capitalize on emerging opportunities and drive market expansion in the Oncology Biosimilar Treatment sector.
North America represents a significant market for oncology biosimilar treatments, driven by robust healthcare infrastructure, high cancer incidence rates, and favorable regulatory frameworks. Regulatory bodies like the FDA, which have program to speed up biosimilar approval procedures and promote market competition, have a significant influence on the biosimilar landscape in oncology.
Report scope:
| Attribute | Details |
| Market Size 2024 | US$ 5.1 Billion |
| Projected Market Size 2034 | US$ 14.7 Billion |
| CAGR Growth Rate | 11.20% |
| Base year for estimation | 2023 |
| Forecast period | 2024 – 2034 |
| Market representation | Revenue in USD Million & CAGR from 2024 to 2034 |
| Market Segmentation | By Product - Monoclonal Antibody, Immunomodulators and Others By Cancer – Blood Cancer, Breast Cancer, Lung Cancer, Cervical Cancer, Colorectal cancer, Kidney cancer, and Others By Distribution Channel - Hospital Pharmacies, Online Pharmacies, Retail Pharmacies |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key highlights of the Oncology Biosimilar Treatment Market:
- The oncology biosimilar treatment market is experiencing rapid growth driven by increasing cancer incidence rates, demand for cost-effective therapies, and patent expirations of reference biologics.
- The focused market is witnessing a growing portfolio of oncology biosimilar treatments targeting various cancer types, including breast cancer, colorectal cancer, lung cancer, and leukemia. Monoclonal antibodies and targeted therapies are among the most prominent classes of biosimilar oncology drugs.
- Oncology biosimilars offer cost-effective alternatives to reference biologics, making cancer treatments more affordable and accessible to patients. Biosimilars contribute to healthcare cost containment efforts and improve patient access to innovative cancer therapies.
- Consolidation among biosimilar manufacturers, strategic alliances, and partnerships with pharmaceutical companies and contract research organizations are reshaping the competitive landscape of the oncology biosimilar market. Collaborations facilitate research and development efforts, regulatory compliance, and market expansion initiatives.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/1096
Explore More Insights:
- IVD Raw Materials Market- Trends, Analysis and Forecast till 2034
- Cell-Based Assay Market– Trends, Analysis and Forecast till 2034
- Bioremediation Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
