Ottawa, Feb. 12, 2024 (GLOBE NEWSWIRE) -- The pharmaceutical stability and storage services market size accounted for USD 3.38 billion in 2024 and is estimated to reach around USD 4.42 billion by 2029, According to Precedence Research. North America led the market with the largest market share of 54% in 2022.
The pharmaceutical stability and storage services market is driven by the growing pharmaceutical sector, the rise in personalized medicine, technological advancements, rising outsourcing trends and others.
The U.S. pharmaceutical stability and storage services market size was USD 1.49 billion in 2023, grew to USD 1.57 billion in 2024, and is expected to surpass around USD 2.49 billion by 2032, growing at a CAGR of 5.6% from 2023 to 2032.
Pharmaceutical stability and storage services are critical aspects of the pharmaceutical industry that focus on maintaining the quality, safety and efficacy of pharmaceutical products throughout their lifecycle. These services ensure that drugs and other medical products remain stable, potent, and safe for use from the time of manufacturing through distribution, storage and until they reach the end-user. The growing investment is expected to drive the pharmaceutical stability and storage services industry during the forecast period.
- For instance, after investing more than USD 1.25 million, the Advanced Therapeutic Medicinal Products (ATMP) and small molecule diagnostics provider, Symbiosis Pharmaceutical Services, a specialized Contract Development and Manufacturing Organization (CDMO), has introduced new internal analytical and microbiological capabilities. Completely approved by the UK Regulatory Agency (MHRA), this service offering expansion will strategically improve the business's primary sterile manufacturing service (also known as "fill/finish") and offer a comprehensive internal Quality Control (QC) function to support Symbiosis's aseptic product manufacturing.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/2632
Key Insights:
- The stability segment is expected to dominate the market during the forecast period.
- The small molecule segment is expected to capture a significant market share over the forecast period.
- The in-house segment held the largest market share in 2023 of more than 60% and is expected to continue the same pattern during the forecast period.
Global Pharmaceutical Stability and Storage Services Market, By Region, 2022-2032 (USD Million)
| Region | 2022 | 2023 | 2027 | 2032 |
| North America | 1,649.10 | 1,735.50 | 2,148.90 | 2,867.30 |
| Europe | 616.3 | 650.4 | 813.9 | 1,100.20 |
| Asia Pacific | 597.3 | 629.3 | 782.8 | 1,050.30 |
| LAMEA | 192.8 | 196.2 | 210 | 226 |
- North America is expected to hold a significant market share during the forecast period. The market growth in the region is attributed to the growing biopharmaceutical sector. The biopharmaceutical sector in North America is significant, with a high number of biotech and pharmaceutical companies developing and manufacturing biologics. These products often have specific storage requirements, driving the demand for specialized stability and storage services.
- For instance, according to recently disclosed statistics on the US bioscience sector, the Biotechnology Innovation Organization (BIO) and the Council of State Bioscience Associations (CSBA) estimated its value at USD 2.9 trillion in 2021. According to the research, nearly 127,000 enterprises in the US industry employed 2.1 million people in 2021.
- Over the anticipated period, the need for pharmaceutical stability and storage services will also be driven by the rising prevalence of uncommon disorders and specialized medications. A sickness or condition that affects fewer than 200,000 Americans is considered a rare disorder, according to the National Organization for Rare Disorders, Inc. Overall, about 30 million Americans suffer from more than 7,000 rare diseases. Thus, the aforementioned statistics are expected to propel the market expansion during the forecast period.
The North America pharmaceutical stability and storage services market size was reached at USD 1.73 billion in 2023 and is anticipated to reach around USD 2.86 billion by 2032.
The Europe pharmaceutical stability and storage services market size was valued at USD 650.4 million in 2023 and is projected to hit around USD 1,100.2 million by 2032.
The Asia Pacific pharmaceutical stability and storage services market size surpassed USD 597.3 million in 2023 and is expected to be worth around USD 1,050.3 million by 2032.
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
Pharmaceutical Stability and Storage Services Market Scope
| Report Coverage | Details |
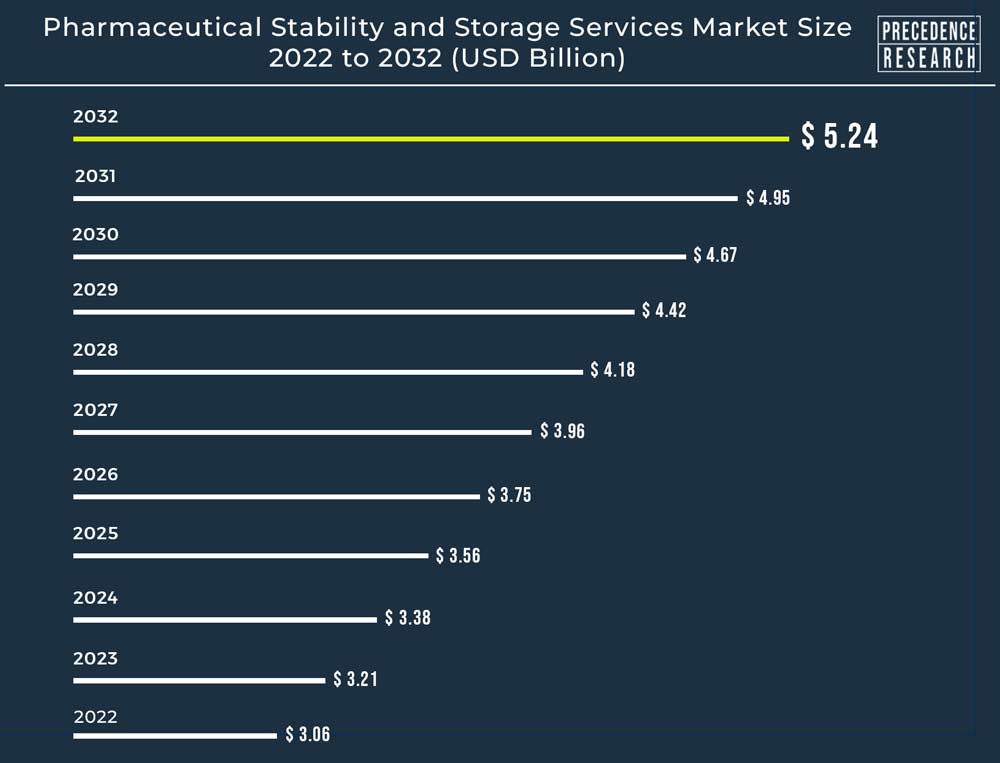
| Global Market Size in 2023 | USD 3.21 Billion |
| Global Market Size by 2032 | USD 5.24 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 5.6% |
| U.S. Market Size in 2023 | USD 1.49 Billion |
| U.S. Market Size by 2032 | USD 2.49 Billion |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Services, By Molecule, By Mode, and Geography |
Pharmaceutical Stability and Storage Services Market Report Highlights:
Global Pharmaceutical Stability and Storage Services Market, By Services, 2022-2032 (USD Million)
| Services | 2022 | 2023 | 2027 | 2032 |
| Stability | 1,976.60 | 2,080.10 | 2,575.10 | 3,435.10 |
| Storage | 1,078.90 | 1,131.30 | 1,380.60 | 1,808.80 |
The stability segment is expected to dominate the market during the forecast period. Stability testing involves subjecting pharmaceutical products to specific environmental conditions such as temperature, humidity and light over a designated period.
The aim is to assess the product quality properties variations over time. These tests are also provided by specialized facilities to pharmaceutical companies. These facilities create controlled environments and conduct studies to determine the shelf life, storage conditions, and packaging requirements for different drug formulations. Thereby, driving the segment expansion.
Global Pharmaceutical Stability and Storage Services Market, By Molecules, 2022-2032 (USD Million)
| Molecules | 2022 | 2023 | 2027 | 2032 |
| Small Molecule | 1,676.50 | 1,763.90 | 2,182.30 | 2,908.70 |
| Large Molecule | 1,379.00 | 1,447.40 | 1,773.40 | 2,335.10 |
The small molecule segment is expected to capture a significant market share over the forecast period. Continuous monitoring of storage conditions is essential for small molecule drugs. Real-time monitoring systems are employed to ensure that temperature, humidity and other environmental factors remain within specific limits. Additionally, the development of small molecule drugs has come a long way in the past thirty years. Approximately 90% of all pharmaceutical medications are small molecules. Fever, migraines, cancer, diabetes, and other prevalent diseases are all treated with it. The need for safe testing and storage of small-molecule pharmaceuticals is being driven by their application in the treatment of prevalent diseases and conditions.
Global Pharmaceutical Stability and Storage Services Market, By Modes, 2022-2032 (USD Million)
| Modes | 2022 | 2023 | 2027 | 2032 |
| In-house | 1,816.80 | 1,911.90 | 2,366.90 | 3,157.20 |
| Outsourcing | 1,238.70 | 1,299.40 | 1,588.80 | 2,086.70 |
The in-house segment held the largest market share in 2023 of more than 60% and is expected to continue the same pattern during the forecast period. The segment expansion is attributed to the advantages of in-house stability services. These services provide companies with direct control and oversight of stability testing processes and conditions. In addition, companies can make real-time decisions regarding product stability, allowing for swift adjustments to formulations, packaging or storage conditions.
Customize this study as per your requirement@ https://www.precedenceresearch.com/customization/2632
Market Dynamics:
Driver: Increasing demand for biosimilars
The market for biosimilars has grown dramatically in recent years because of their similarity to biologics and generally cheaper cost. The use of biosimilars in the treatment of autoimmune disorders, cancer, and other chronic illnesses has grown significantly. Due to the high frequency of these diseases around the world, there will likely be an increase in demand for biosimilars as well as a need for their stability and storage. Because different regulatory bodies have varying data requirements and testing criteria for evaluating a product's stability, marketing items throughout the market can be difficult. This is expected to raise the need for outsourcing services in the industry.
Restraint: High investment and complex regulatory landscape
Establishing and maintaining state-of-the-art stability and storage facilities require significant capital investment. This can be a restraint, especially for smaller pharmaceutical companies with limited financial resources. In addition, compliance with stringent regulatory requirements, including Good Manufacturing Practices (GMP) and other guidelines, can be challenging and resource intensive. Meeting regulatory standards for storage and stability testing necessitates ongoing investments in technology, personnel, and infrastructure. Therefore, the high investment and complex regulatory landscape might be a major restraining factor for the market expansion.
Opportunity: Growing complexity of pharmaceuticals
The pharmaceutical industry is evolving with the development of more complex and sensitive drug formulations. Specialized storage and stability services are required to meet the unique needs of these products, which may be susceptible to temperature fluctuations, light exposure, or other environmental factors. Thus, these complexities in the pharmaceutical industry are expected to offer a lucrative opportunity for the pharmaceutical stability and storage services market during the forecast period.
Related Reports:
- Pharma 4.0 Market: The global pharma 4.0 market size was valued at USD 12.05 billion in 2022 and is expected to be worth around USD 63.17 billion by 2032, growing at a CAGR of 18.02% from 2023 to 2032.
- Small Molecule Immunomodulators Market: The global small molecule immunomodulators market size was estimated at USD 156.2 billion in 2022 and it is predicted to reach around USD 283.70 billion by 2032, growing at a CAGR of 6.20% from 2023 to 2032.
- Small Molecule Drug Discovery Market: The global small molecule drug discovery market size reached USD 75.96 billion in 2022 and is projected to hit around USD 163.76 billion by 2032, registering a CAGR of 7.97% during the forecast period from 2023 to 2032.
- Small Molecule API Market: The global small molecule API market size was exhibited at USD 176 billion in 2022 and is projected to hit around USD 299.2 billion by 2032, growing at a CAGR of 5.5% during the forecast period from 2023 to 2032.
- Artificial Intelligence (AI) In Drug Discovery Market: The global artificial intelligence (AI) in drug discovery market size was valued at USD 1.4 billion in the year 2022 and is expected to hit around USD 9.7 billion by 2032 with an increasing CAGR of 21.36% during the forecast period 2023 to 2032.
Recent Developments:
- In October 2022, Cambrex announced the growth of Q1 Scientific, its stability storage division that provides pharmaceutical, medical device, and life sciences businesses with environmentally controlled stability storage services. Q1 Scientific plans to expand its Waterford, Ireland, location by 10,000 square feet and construct a new 20,000-square-foot cGMP facility in Belgium. Situated around 50 miles from Brussels in the conveniently accessible Liege region, Q1 Scientific's new state-of-the-art temperature-controlled and monitored storage facility will be built in Belgium. To facilitate long-term, intermediate, and expedited stability studies, the facility will offer 35,000 temperature-controlled storage rooms for all major ICH climatic zones, including 2–8°C and 25°C / 60%RH and 40°C / 75%RH.
- In October 2023, the range of high-performing pharmaceutical component solutions from Clariant, a specialty chemicals firm with a focus on sustainability, has been expanded to include new products that will aid in the development of safe and efficient medications. To position itself as the industry's one-stop shop for solutions, Clariant will introduce three new VitiPure® excipients at CPHI Barcelona. These excipients enable a variety of Active Pharmaceutical Ingredient (API) formulations and administration routes, even for sensitive ones like mRNA vaccines and biological medications.
Market Key Players:
- Catalent, Inc.
- Charles River Laboratories International, Inc
- Almac Group
- Eurofins Scientific
- Lucideon Limited
- Intertek Group Plc
- Alcami Corporation
- Element Materials Technology
- Q1 Scientific
- BioLife Solutions
- Masy BioServices
- Roylance Stability Storage Limited
- Reading Scientific Services Ltd.
- Als Ltd.
- Auriga Research Private Limited
Market Segmentation
By Services
- Stability
- Drug Substance
- Stability Indicating Method Validation
- Accelerated Stability Testing
- Photostability Testing
- Others
- Storage
- Cold
- Non-cold
By Molecule
- Small Molecule
- Research Products
- Commercial Products
- Large Molecule
- Research Products
- Commercial Products
By Mode
- In-house
- Outsourcing
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
You can place an order or ask any questions, please feel free to contact us at
sales@precedenceresearch.com | +1 650 460 3308
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/2632
Unlocking Market Insights through Data Excellence
The "Precedence Statistics" flexible dashboard is a powerful tool that offers real-time news updates, economic and market forecasts, and customizable reports. It can be configured to support a wide range of analysis styles and strategic planning needs. This tool empowers users to stay informed and make data-driven decisions in various scenarios, making it a valuable asset for businesses and professionals looking to stay ahead in today's dynamic and data-driven world.
To Access our Premium Real-Time Data Intelligence Tool, Visit:
www.precedencestatistics.com
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com/
Our Blogs:
https://www.towardshealthcare.com
https://www.towardspackaging.com
For Latest Update Follow Us:
