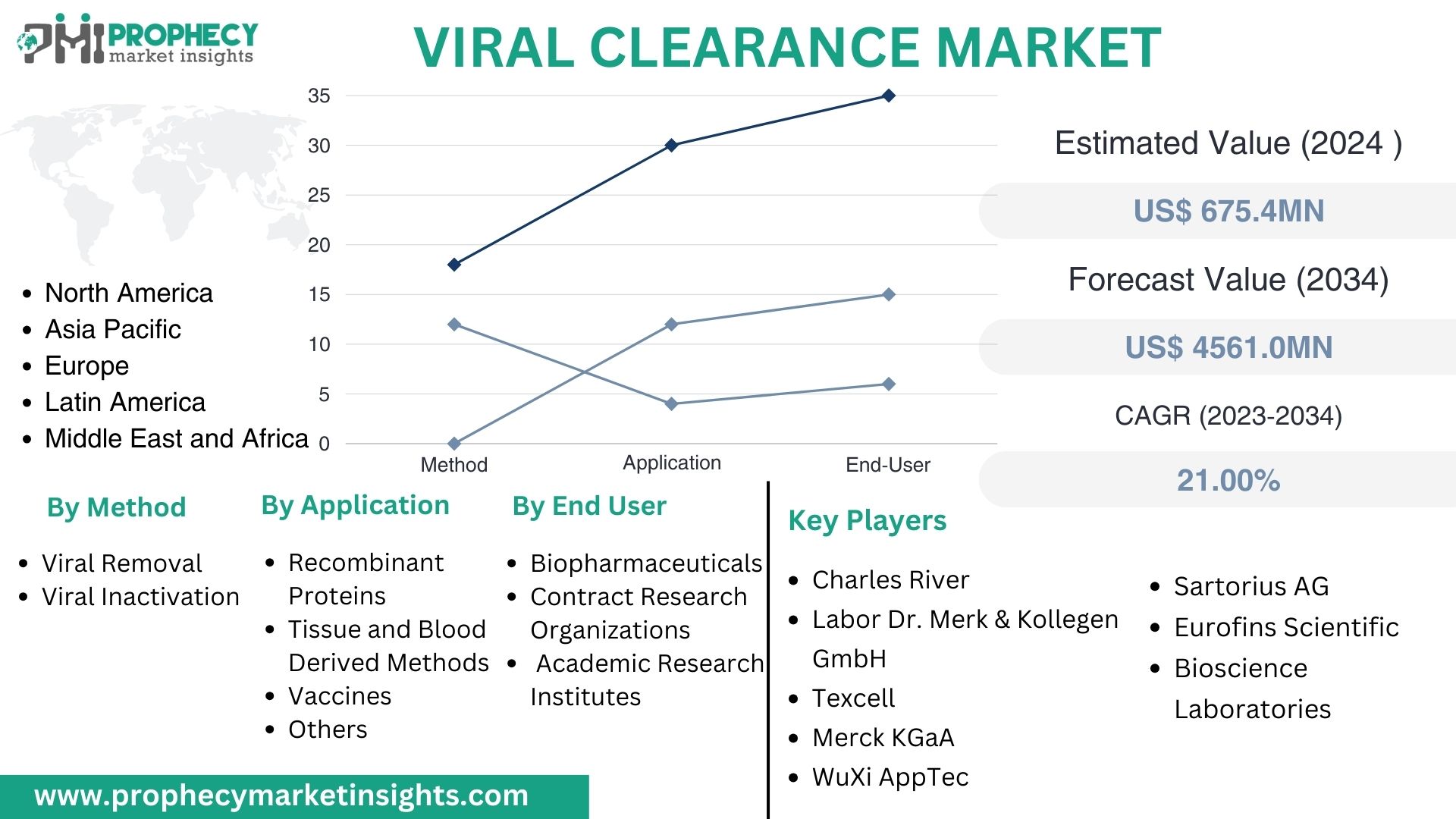
Covina, Feb. 15, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the Viral Clearance Market size was valued at about USD 675.4 Million in 2024 and expected to grow at CAGR of 21.00% to extend a value of USD 4561.0 Million by 2034.”
What is Viral Clearance?
- Market Overview:
Viral clearance refers to the process by which viruses are removed or reduced to acceptable levels in biological products, such as vaccines, therapeutic proteins, blood products, and other biopharmaceuticals. This process is essential to ensure the safety and efficacy of these products for human use. Viral clearance involves a series of steps and methods designed to eliminate or inactivate viruses that may contaminate the biological material during the manufacturing process.
Viral clearance is a critical aspect of the manufacturing process for biological products, particularly those derived from human or animal sources, as it helps mitigate the risk of transmitting infectious agents to patients receiving the products. Regulatory authorities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require thorough documentation of viral clearance procedures and validation data as part of the approval process for biopharmaceutical products.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/3912
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Top Leading Players in Viral Clearance Market:
- Labor Dr. Merk & Kollegen GmbH
- Merck KGaA
- WuXi AppTec
- Sartorius AG
- Eurofins Scientific
- Bioscience Laboratories
- Syngene
- Vironova
- Sigma-Aldrich Corporation
- Wuxi Biologics (Cayman) (China)
- Merck KGaA (Germany)
- Charles River Laboratories International Inc. (US)
- Texcell Inc.
Market Dynamics:
Driving Factors:
- The growing demand for biopharmaceuticals, including vaccines, therapeutic proteins, and monoclonal antibodies, is driving the need for effective viral clearance methods to ensure product safety.
- Regulatory agencies such as the FDA and EMA impose strict guidelines for the clearance of viruses in biopharmaceutical products, necessitating the adoption of advanced viral clearance technologies and processes.
- The prevalence of viral infections, including emerging viruses and known pathogens, underscores the importance of robust viral clearance measures to prevent transmission through biological products.
- Ongoing advancements in filtration techniques, chromatography, chemical inactivation methods, and other viral clearance technologies contribute to the growth of the viral clearance market by offering more efficient and reliable solutions.
- Pharmaceutical companies and biotechnology firms are investing heavily in research and development to enhance viral clearance technologies, develop novel products, and improve manufacturing processes thereby driving market growth.
Restrain Factors:
- High Cost of Viral Clearance Technologies
- Complexity of Regulatory Compliance
- Potential Impact on Product Yield and Quality
Emerging Trends and Opportunities in Viral Clearance Market:
- Outsourcing viral clearing services is becoming more and more popular as biopharmaceutical companies concentrate on creating cutting-edge treatments and vaccines. Viral clearance-focused contract research organisations (CROs) present chances for market expansion by providing infrastructure and experience to enable the creation and validation of viral clearance procedures.
- The biopharmaceutical sector is starting to adopt single-use viral clearance systems more frequently because of their adaptability, affordability, and lower danger of cross-contamination. To improve operational efficiency and expedite virus clearance procedures, manufacturers are spending more in single-use technology like as chromatography columns and disposable filtration systems.
- Advances in bioprocessing and molecular biology are driving the development of novel viral clearance technologies capable of addressing complex viral challenges. Innovative approaches such as nanofiltration, affinity chromatography, and viral inactivation using novel agents are being explored to improve the efficiency, specificity, and scalability of viral clearance methods.
- Continuous manufacturing offers significant advantages in terms of process control, product quality, and efficiency compared to traditional batch processing. The integration of continuous viral clearance technologies into biopharmaceutical manufacturing workflows presents opportunities to enhance productivity, reduce cycle times, and optimize resource utilization while ensuring viral safety.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/3912
Challenges of Viral Clearance Market:
- Questions concerning the safety of viruses and the efficiency of viral clearance procedures can have an impact on public opinion and confidence in biopharmaceutical products. To preserve consumer trust and reduce reputational risks, manufacturers must be open and honest about their procedures for virus clearance, compliance with legal requirements, and dedication to product safety.
- In order to meet the increasing demand for viral clearing services, the sector must have enough capacity and scalability. In order to accommodate growing volumes of biopharmaceutical products while upholding strict standards of virus safety and regulatory compliance, manufacturers need to make investments in infrastructure, personnel, and operational skills.
Detailed Segmentation:
Viral Clearance Market, By Type:
-
-
- Viral Removal
- Viral Inactivation
-
Viral Clearance Market, By Application:
-
-
- Recombinant Proteins
- Tissue and Blood Derived Methods
- Vaccines
- Others
-
Viral Clearance Market, By End-User:
-
-
- Biopharmaceuticals
- Contract Research Organizations
- Academic Research Institutes
-
Viral Clearance Market, By Region:
-
-
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
-
- Asia Pacific
-
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Mexico
- Rest of Latin America
-
- Middle East & Africa
-
- GCC
- Israel
- South Africa
- Rest of Middle East & Africa
-
- North America
-
Regional Analysis:
Regional insights highlight the diverse market dynamics, regulatory landscapes, and growth drivers shaping the Viral Clearance Market across different geographic areas. Understanding regional nuances and market trends is essential for stakeholders to capitalize on emerging opportunities and drive market expansion in the Viral Clearance sector.
North America is estimated to witness a huge market growth as the Food and Drug Administration (FDA) in the United States and Health Canada in Canada oversee the tight regulatory environment in North America, which places a great deal of emphasis on viral safety criteria for biopharmaceutical products. The demand for cutting-edge viral clearance technology and knowledge is driven by biopharmaceutical manufacturers operating in the region placing a high focus on complying with regulatory criteria for viral clearance.
Report scope:
| Attribute | Details |
| Market Size 2024 | US$ 675.4 Million |
| Projected Market Size 2034 | US$ 4561 Million |
| CAGR Growth Rate | 21.00% |
| Base year for estimation | 2023 |
| Forecast period | 2024 – 2034 |
| Market representation | Revenue in USD Million & CAGR from 2024 to 2034 |
| Market Segmentation | By Method - Viral Removal And Viral Inactivation By Application - Recombinant Proteins, Tissue And Blood Derived Methods, Vaccines, Others By End-User - Biopharmaceuticals, Contract Research Organizations, And Academic Research Institutes |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key highlights of the Viral Clearance Market:
- The increasing demand for biopharmaceutical products, including monoclonal antibodies, vaccines, recombinant proteins, and cell and gene therapies, is driving the growth of the viral clearance market. As the biopharmaceutical pipeline expands and new therapies emerge, the need for effective viral clearance processes becomes more pronounced.
- Regulatory agencies such as the Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in Europe, and other regulatory bodies worldwide impose strict guidelines for viral safety in biopharmaceutical products. Compliance with regulatory standards for viral clearance is essential for product approval and market acceptance.
- The viral clearance market continues to benefit from technological advancements and innovation in areas such as filtration, chromatography, viral inactivation, and molecular biology. These advancements enable the development of more efficient, specific, and scalable viral clearance methods, enhancing product safety and manufacturing efficiency.
- Many biopharmaceutical companies outsource viral clearance testing and validation activities to specialized contract research organizations (CROs) and testing laboratories. Outsourcing allows companies to access specialized expertise, infrastructure, and resources while focusing on core competencies such as drug development and commercialization.
- The growing adoption of cell and gene therapies presents unique challenges for viral safety due to the use of viral vectors and genetically modified organisms. Viral clearance plays a critical role in ensuring the safety and efficacy of cell and gene therapy products by mitigating the risk of viral vector contamination and adventitious agent transmission.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/3912
Explore More Insights:
- Bioreactors and Fermenters Market- Trends, Analysis and Forecast till 2034
- Downstream Polyethylene Market– Trends, Analysis and Forecast till 2034
- Chemotherapy Induced Acral Erythema (Hand Foot syndrome) Treatment Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
