Dublin, Feb. 27, 2024 (GLOBE NEWSWIRE) -- The "Asia-Pacific Cell and Gene Therapy Manufacturing QC Market: Analysis and Forecast, 2023-2033" report has been added to ResearchAndMarkets.com's offering.
The Asia-Pacific cell and gene therapy manufacturing QC market was valued at $426.3 million in 2023 and is expected to reach $2,233.6 million by 2033, growing at a CAGR of 18.01% during the forecast period 2023-2033.
The cell and gene therapy manufacturing quality control (QC) market is projected to grow due to the rising number of approved therapies and the need for expanded infrastructure. Furthermore, the broader range of medical conditions targeted by cell and gene therapies necessitates large-scale manufacturing and QC processes, contributing to market growth.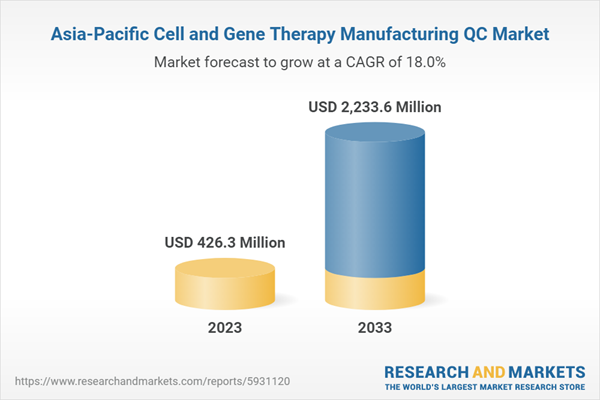
The Asia-Pacific (APAC) region is witnessing significant growth in the cell and gene therapy manufacturing quality control (QC) market. This expansion is driven by several factors, including the rising adoption of advanced therapies, increasing investments in biotechnology and pharmaceutical sectors, and the presence of a skilled workforce.
Moreover, the growing prevalence of chronic diseases in the region has accelerated the demand for innovative cell and gene therapies, necessitating stringent QC measures. APAC countries are actively involved in research and development activities, fostering collaborations with biotech companies. As a result, the APAC cell and gene therapy manufacturing QC market is poised for substantial growth in the coming years, contributing to the advancement of healthcare solutions in the region.
How can this report add value to an organization?
Workflow/Innovation Strategy: The APAC cell and gene therapy manufacturing QC market (by offering) has been segmented into products and services. Moreover, the study provides the reader with a detailed understanding of the different applications of cell and gene therapy manufacturing QC in raw material preparation, upstream processing, downstream processing, and packaging.
Growth/Marketing Strategy: Cell and gene therapy manufacturing QC is being used for raw material preparation, upstream processing, downstream processing, and packaging. Various companies are providing products and services to aid in the manufacturing and QC of various cell and gene therapies, which is also the key strategy for market players to excel in the current APAC cell and gene therapy manufacturing QC market.
Competitive Strategy: Key players in the APAC cell and gene therapy manufacturing QC market have been analyzed and profiled in the study, including manufacturers involved in new product launches, acquisitions, expansions, and strategic collaborations. Moreover, a detailed competitive benchmarking of the players operating in the APAC cell and gene therapy manufacturing QC market has been done to help the reader understand how players stack against each other, presenting a clear market landscape.
Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 101 |
| Forecast Period | 2023 - 2033 |
| Estimated Market Value (USD) in 2023 | $426.3 Million |
| Forecasted Market Value (USD) by 2033 | $2233.6 Million |
| Compound Annual Growth Rate | 18.0% |
| Regions Covered | Asia Pacific |
Key Topics Covered:
Executive Summary
1 Markets
1.1 Product Definition
1.2 Market Scope
1.2.1 Key Questions Answered in the Report
1.3 Research Methodology
1.4 Market Overview
1.4.1 Market Scenario
1.4.2 Market Footprint and Growth Potential
1.4.3 Future Potential
1.4.4 COVID-19 Impact on Market
1.4.4.1 Impact on Research and Clinical Operations
1.4.4.2 COVID-19 Impact: Current Scenario of the Market
1.4.4.3 Pre- and Post-COVID-19 Impact Assessment
1.4.4.3.1 Pre-COVID-19 Phase
1.4.4.3.2 Post-COVID-19 Phase
2 Cell and Gene Therapy Manufacturing QC Market: Industry Analysis
2.1 Regulatory Framework
2.1.1 Chemistry, Manufacturing, and Control (CMC) Requirements by the Food and Drug Administration (FDA)
2.1.1.1 Product Testing
2.1.1.2 Microbial Testing
2.1.1.3 Identity
2.1.1.4 Purity
2.1.1.5 Potency
2.1.1.6 Viability
2.1.1.7 Cell Number or Dose
2.1.2 Quality Aspects of Cell and Gene Therapy Products by the European Medicines Agency (EMA)
2.1.2.1 Characterization
2.1.2.2 Identity Testing
2.1.2.3 Purity Testing
2.1.3 Current Good Manufacturing Practice (CGMP) Regulations
2.1.4 Regulatory Framework: Cell and Gene Therapy Manufacturing QC Market
3 Cell and Gene Therapy Manufacturing QC Market (by Region)
3.1 Overview
3.2 Asia-Pacific
3.2.1.1 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering)
3.2.1.2 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
3.2.1.3 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
3.2.1.4 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
3.2.1.5 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
3.2.2 China
3.2.3 Japan
3.2.4 South Korea
3.2.5 Australia
3.2.6 India
3.2.7 Rest-of-Asia-Pacific
4 Company Profiles
4.1 Overview
4.2 Manufacturers
4.2.1 WuXi AppTec
4.2.1.1 Company Overview
4.2.1.2 Role of WuXi AppTec in the Cell and Gene Therapy Manufacturing QC Market
4.2.1.3 Key Competitors
4.2.1.4 Financials
4.2.1.5 Analyst Perspective
For more information about this report visit https://www.researchandmarkets.com/r/3ctwj3
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
