TRUMBULL, Conn., April 21, 2022 (GLOBE NEWSWIRE) -- As businesses return to normal operations with much more limited staffing, they can also expect to see workplace injuries increase, requiring employees to get specialized medical treatments or devices - which may be delayed or unavailable due to peaking supply chain logjams and regulatory challenges. ZetrOZ Systems, makers of the sustained acoustic medicine sam® device, is prepared to meet patients' needs through careful advanced planning and multi-site U.S. manufacturing.
ZetrOZ's sam® is the only FDA-cleared, long-duration, home-use, ultrasound device to increase local circulation and heal pain, and is designed for easy daily use as necessary. More than 30 peer-reviewed studies have found it to be an innovative and effective treatment to promote injury healing, relieve pain, restore function, and return patients to work, sports and other daily activities. Its low-intensity continuous ultrasound therapy treats soft tissue injury by inhibiting inflammation and increasing the rate of tissue regeneration, angiogenesis, and nutrient exchange.
Dr. George Lewis, president of ZetrOZ Systems, said one key for his company meeting rising demand was assessing manufacturing processes to look for potential problems in assembly, sub-assembly, or process components.
"Sometimes simple things like glues or screws can be very specific to a manufacturing process, and in some cases, not having the right adhesive to glue two parts together can set you back months at a time," Lewis said. "Sometimes a piece of precision manufacturing equipment, like a laser welder, is the culprit."
In March, the Logistics Managers Index indicated that supply chain pressures have reached record levels, with costs surging and warehouse capacity at a minimum. Those pressures have ZetrOZ assessing potential one-source and one-step failure points weekly, Lewis said.
ZetrOZ's custom manufacturing formulations, processes, and parts also require the company to source its manufacturing one to two years in advance. With sam® medical products being manufactured in the United States, production does not rely on shipping backlogs or harbor ports of entry of finished products, making it more sustainable, Lewis said.
That allows ZetrOZ to pre-stock inventory for production, which may be challenging for small manufacturers of medical devices who may be unable to fund the associated costs of these actions until their products are sold.
The importance of ZetrOZ's preparations is highlighted by U.S. Chamber of Commerce reports, which found that almost 60% of small businesses reported disruptions and alterations in 2021 due to supply chain and other issues.
Medical devices are particularly vulnerable, Lewis said, because it is near-impossible to bring alternatives to market quickly. Medical device development typically takes years and tens to hundreds of millions of dollars in research and development. Then, the Food & Drug Administration's strict and thorough review and approval process for class II and class III medical devices can take several months or years, and then device makers still have to develop manufacturing processes.
Visit samrecover.com to learn more about the sam® ultrasound device. Learn more about ZetrOZ Systems a zetroz.com.
Media Contact:
Alexandra Batista
alexandra@newswire.com
Related Images
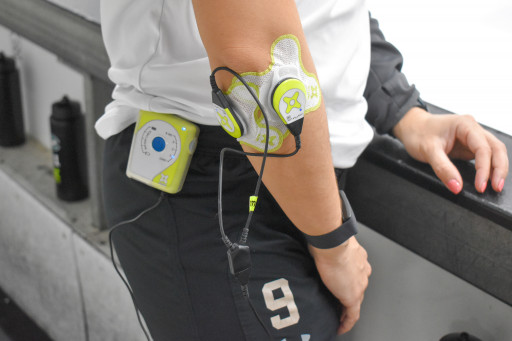
Image 1
Made in the USA, sam medical devices are used to help injured patients heal without surgery and medication.
This content was issued through the press release distribution service at Newswire.com.
Attachment
