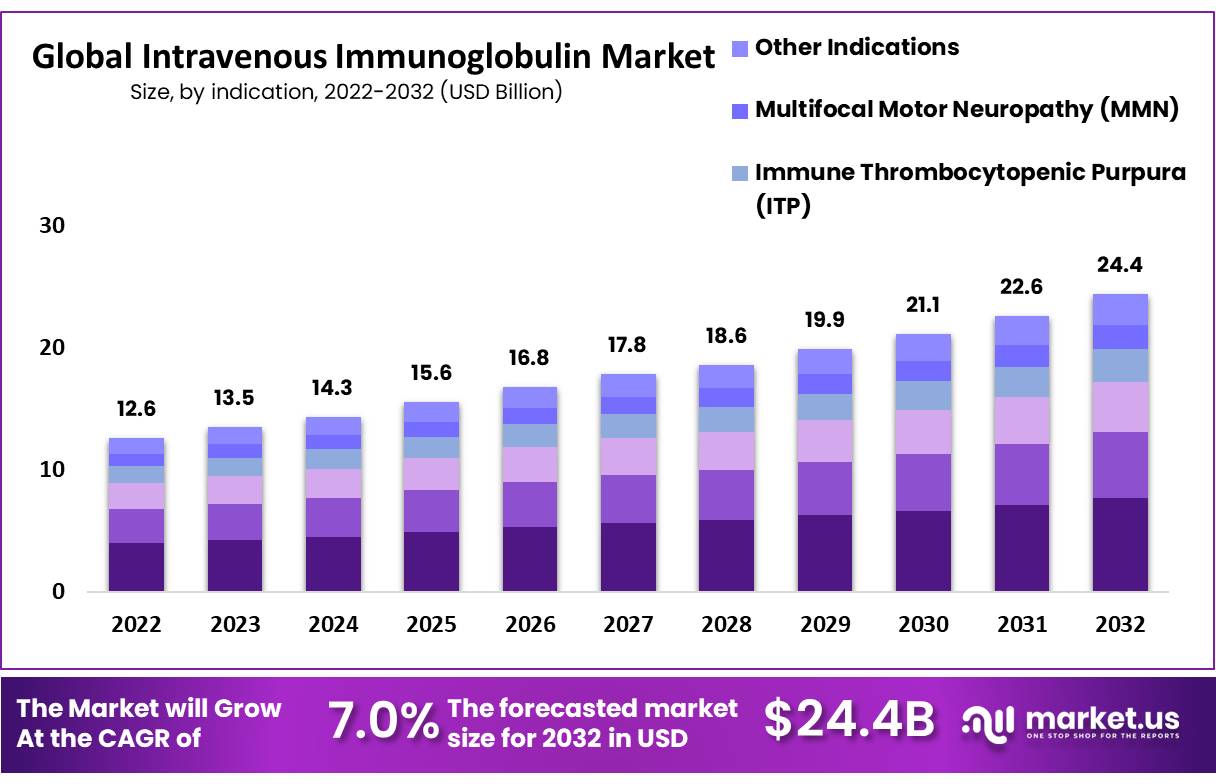
New York, May 22, 2023 (GLOBE NEWSWIRE) -- The latest report on the Intravenous Immunoglobulin Market estimates register an incremental growth of USD 24.4 Billion by 2032 from USD 12.6 Billion in 2022 at a CAGR of 7% during the forecast period.
Market drivers include the growing elderly population, the rising prevalence of immunodeficiency disorders, the increasing use of intravenous immunoglobulin treatments (IVIG), and the rising use of non-label indications. IVIG preparations have been developed primarily because of the rising incidence of immunodeficiency disorder patients.
To Get Additional Highlights On Major Revenue-Generating Segments, Request an Intravenous Immunoglobulin Market Sample Report At https://market.us/report/intravenous-immunoglobulin-market/request-sample/
Key Takeaway
- By indication, the primary immunodeficiency segment generated a revenue share of 31.6% in 2022.
- By distribution channel, the Hospital pharmacies segment has dominated the market and generated a revenue share of 57.3% in 2022.
- In 2022, North America dominated the market with the highest revenue share of 49.5%.
- The Asia Pacific region is expected to experience a significant growth rate during the forecast period.
Injectable immunoglobulin therapy (IVIG) has been a major primary immunodeficiency disease (PID) treatment over the last few years. PIDs that have antibody deficiency are treated with a variety of medical treatments. These PIDs account for over half of all primary immunodeficiencies. These diseases, including common variable immunodeficiency (CVID), X-linked agammaglobulinemia, and others, are partially characterized by a deficiency or impaired antibody function. This is due to the high prevalence of primary immunodeficiency, a key factor in driving this segment's growth.
It is expensive to diagnose and treat demyelinating diseases. The rising cost of IVIG therapy hinders market expansion. Infusions of immunoglobulin are usually given every 3-4 weeks. The therapy lasts approximately 12-16 sessions per year. IVIG costs USD 73.89 per Gram and can cost USD 10,000 depending on the severity or condition of the patient.
Factors affecting the growth of the Intravenous immunoglobulin industry?
Some factors have contributed to the growth of the intravenous immunoglobulin industry are-
- Government Regulations- Strict government regulations regarding intravenous immunoglobulin product use and the high risk of side effects will impede the market's growth.
- Increasing Geriatric Population- The increase in the geriatric population and the number of hemophilics, better technologies for immunoglobulin production, and improved purification techniques (with a higher plasma yield) are helping the market grow.
To Understand How Our Report Can Bring A Difference To Your Business Strategy, Inquire About a Brochure At https://market.us/report/intravenous-immunoglobulin-market/#inquiry
Top Trends in Global Intravenous Immunoglobulin Market
Injectable immunoglobulin therapy (IVIG) has been a major primary immunodeficiency disease (PID) treatment over the last few years. PIDs that have antibody deficiency are treated with a variety of medical treatments. These PIDs account for over half of all primary immunodeficiencies. These diseases, including common variable immunodeficiency (CVID), X-linked agammaglobulinemia, and others, are partially characterized by a deficiency or impaired antibody function. This is due to the high prevalence of primary immunodeficiency, a key factor in driving this segment's growth.
Several pharmaceutical companies also develop immunoglobulin products to treat primary immunodeficiency. In May 2021, GC Pharma conveyed to the FDA that it had taken the company's Biologics License Application. This was for "GC5107" (Immune Globulin Intravenous), intended for treating Primary Humoral Immunodeficiency. This is a group of inherited genetic conditions that cause a person to have a weak or absent immune technique.
The study segment will grow with IVIG therapies available and improvements in intravenous immunoglobulin products to treat primary immunodeficiency disease, which is highly probable.
Market Growth
Many factors have contributed to the growth of the intravenous immunoglobulin market. There has been a significant rise in the elderly population, the number of hemophilics, and better technology for producing immunoglobulin. Also, purification techniques have improved with a higher plasma yield. The rise will also influence market growth in the incidence of chronic inflammatory demyelinating and other diseases, such as hypogammaglobinaemia (CIDP) and others. Strict government regulations on intravenous immunoglobulin products and high risks of side effects will limit the market growth. Intravenous immunoglobulins can be used to treat many diseases and are expected to offer lucrative opportunities.
Regional Analysis
The intravenous immunoglobulin market was dominated by North America, with a revenue share of 49.5%. Market growth is attributed to rising awareness of products used to treat immunodeficiency disease, growing interest among clinicians, and rising healthcare spending. The growth of the marketplace is anticipated to be aided by the increasing use of IVIG therapies to treat disorders.
Competitive Landscape
The competitive landscape of the market has also been examined in this report. Some of the major players include CSL Behring, Shire (Takeda Pharmaceutical Company Limited), Grifols, S.A., Kedrion S.p.A, Octapharma, Bio Products Laboratory Ltd., Biotest AG, China Biologic Products Holdings, Inc., LFB SA, Shanghai RAAS Blood Products Co., Ltd., etc.
Scope of the Report
| Report Attributes | Details |
| Market Size in 2022 | US$ 12.6 Billion |
| Market Size in 2032 | US$ 24.4 Billion |
| CAGR | 7.0% |
| North America Revenue Share | 49.5% |
| Base Year | 2022 |
| Historic Period | 2023 to 2032 |
| Forecast Period | 2023 to 2032 |
Market Drivers
Increase in the geriatric population and the number of hemophiliacs, better technologies for immunoglobulin production, and enhanced purification strategies. Furthermore, the surge in the prevalence of diseases like hypogammaglobulinemia, chronic inflammatory demyelinating polyneuropathy (CIDP), and others is expected to boost market growth.
Market Restraints
On the other hand, it is costly to diagnose and treat demyelinating diseases. The increasing cost of IVIG treatment hinders market growth. Infusions of immunoglobulin are usually given every 3-4 weeks. The therapy lasts approximately 12-16 sessions per year.
Market Opportunities
Many immunoglobulins have seen increased funding in the market. The national institute of Allergy and infectious diseases funded the phase III trial, Inpatient treatment with anti-coronavirus immunoglobulin. The antibody solution was tested in the ITAC trial as inpatient treatment with anti-coronavirus immunoglobulin.
Grow Your Profit Margin With Market.Us - Purchase This Premium Report at https://market.us/purchase-report/?report_id=101568
Report Segmentation of the intravenous immunoglobulin Market
Indication Insight
One of the key factors driving the intravenous immunoglobulin (IVG) market growth in 2022 is the adoption of intravenous immunoglobulin therapy as a first-line treatment for primary immunodeficiency and other rare immunological or neurological diseases.
The market share for Primary Immunodeficiency is the highest among all indication segments. In 2022, the market share for Primary Immunodeficiency was 31.6%. This is expected to rise over the forecast period. The primary immunodeficiency segment is driven by a growing focus on diagnosing primary immunodeficiency (PI) and subsequent treatment that is based on individual cases and training. Most patients are not skilled in self-administration.
Distribution Channel Insight
Hospital pharmacies dominated the market, with a revenue share exceeding 57.3% in 2022. This is due to the extensive network of hospitals and the wide range of products that can be found in hospital pharmacies. The increasing incidence of primary immune deficiencies, such as hepatitis C and other diseases, has increased hospitalizations worldwide, increasing customer preference for hospital pharmacies. Hospitals offer quick reimbursement, treatment, and proper care to many patients. This has led to an increase in patients choosing hospital pharmacies.
To Get Additional Highlights On Major Revenue-Generating Segments, Request an Intravenous Immunoglobulin Market Sample Report At https://market.us/report/intravenous-immunoglobulin-market/request-sample
Market Segmentation
By Type
- IgG
- IgM
- IgA
- IgE
- IgD
By Indication
- Primary Immunodeficiency
- Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Guillain-Barre Syndrome (GBS)
- Immune Thrombocytopenic Purpura (ITP)
- Multifocal Motor Neuropathy (MMN)
- Hypogammaglobinemia
- Myasthenia Gravis
- Specific antibody deficiency
- Inflammatory myopathies
- Other Indications
By Distribution Channels
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacies
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
These are some of the major players in the global Intravenous Immunoglobulin Market:
- CSL Behring
- Shire (Takeda Pharmaceutical Company Limited)
- Grifols, S.A.
- Kedrion S.p.A
- Octapharma
- Bio Products Laboratory Ltd.
- Biotest AG
- China Biologic Products Holdings, Inc.
- LFB SA
- Shanghai RAAS Blood Products Co., Ltd.
- Other Key Players
Recent Development of the Intravenous Immunoglobulin Market
- In May 2019, The FDA authorized the prior authorization supplement (PAS) for ADMA Biologics, Inc.’s product offering of BIVIGAM, and then ADMA announced that the company started commercial sales.
- In April 2019, A novel intravenous immunoglobulin from ADMA Biologics, Inc. will be used to treat primary immunodeficiency, Asceniv (Immune).
Browse More Related Reports
- Custom Antibody Services Market Scenario is expected to register a growth of significant CAGR of 8.9% during the forecast period (2022–2031)
- TCR-based antibody Market is projected to reach a valuation of USD 12,865.51 Mn by 2032 at a CAGR of 15.20%, from USD 3,125.38 Mn in 2022.
- Dry Eye Syndrome Market size is expected to be worth around US$ 7,940 Mn by 2032 from US$ 4,540 Mn in 2022, growing at a CAGR of 5.9%
- ePharmacy Market is projected to achieve a valuation of USD 2,07,117.88 Mn by 2032 at a CAGR of 16.2%, from USD 46,148.37 Mn in 2022.
About Us:
Market.US (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. Market.US provides customization to suit any specific or unique requirement and tailor-makes reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us On LinkedIn Facebook Twitter
Our Blog:
- https://medicalmarketreport.com/
- https://chemicalmarketreports.com/
- https://techmarketreports.com/
- https://foodnbeveragesmarket.com/
