Dublin, Oct. 16, 2023 (GLOBE NEWSWIRE) -- The "Non-Invasive Prenatal Testing (NIPT) Market, Size, Global Forecast 2023-2030, Industry Trends, Share, Growth, Insight, Impact of Inflation, Company Analysis" report has been added to ResearchAndMarkets.com's offering.
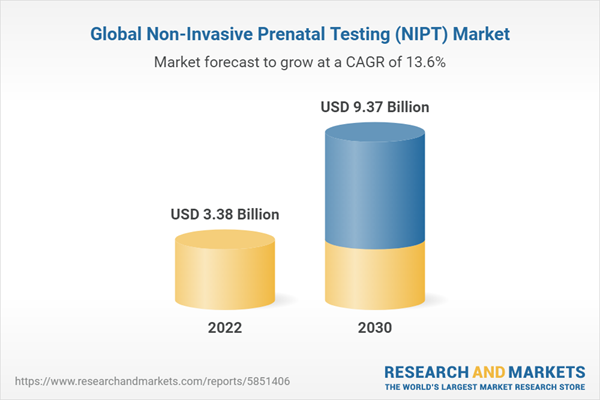
In 2022, the market for non-invasive prenatal testing (NIPT) was worth US$3.38 Billion and is expected to be worth about US$ 9.37 Billion by 2030, growing at a CAGR of 13.6% during 2022-2030
Non-invasive prenatal testing (NIPT) has emerged as a widely adopted screening approach for fetal aneuploidies, with the use of cell-free fetal DNA in maternal plasma revolutionizing prenatal care. This cutting-edge technique has significantly transformed the diagnosis of chromosomal abnormalities during pregnancy, surpassing previous advancements in the field and marking a significant milestone in obstetric medicine.
NIPT represents a sophisticated method for identifying chromosomal abnormalities in developing fetuses, offering expecting mothers highly effective and affordable early detection of genetic diseases. The global NIPT market is experiencing substantial growth, partly attributed to expanded insurance coverage and reimbursement policies worldwide. The increasing prevalence of genetic abnormalities and substantial investments in genome sequencing by the healthcare industry are key drivers of this market expansion.
Recent years have witnessed a shift in prenatal screening towards non-invasive methods, driven by therapeutic benefits and economic advantages. Advanced non-invasive diagnostics have gained clinical utility and are becoming the standard for pregnant women in countries like the Netherlands.
While invasive diagnostics remain relevant for specific conditions, non-invasive technologies have gained widespread acceptance. The rising incidence of chromosomal abnormalities in late pregnancies, coupled with favorable reimbursement options, further fuels the demand for non-invasive fetal diagnostics.
Advancements in genomic sequencing technology, particularly Next-generation sequencing (NGS), have reduced diagnostic complexity and turnaround time, contributing to the rise of NIPT utilizing NGS technology. Life science companies are actively developing NGS-based solutions for the early and accurate diagnosis of genetic disorders, positioning themselves competitively in the market. The increasing need for early and non-invasive fetal diagnostics, coupled with supportive reimbursement regulations, is propelling market growth.
Furthermore, the ability of NIPT to aid in the early detection of illnesses, including chromosomal abnormalities, has become increasingly important. Recent studies indicate a prevalence of 0.4% to 0.9% of babies with chromosomal abnormalities, emphasizing the demand for testing and driving overall market expansion. The NIPT market is positioned to meet this demand and facilitate early disease detection, contributing to its rapid growth.
Cell-free DNA tests are growing in the worldwide NIPT market due to its non-invasive nature, which decreases risks and inconvenience for pregnant women.
Ultrasound detection, biochemical screening tests, cell-free DNA in maternal plasma tests, and others are among the procedures used in the worldwide non-invasive prenatal testing (NIPT) market. Because of advancements in sequencing technology and analytics, very accurate cell-free DNA tests for diagnosing chromosomal abnormalities are becoming more commonly accessible.
Furthermore, rising mother age and the tendency toward later pregnancies have raised need for prenatal screening. Furthermore, the support and recognition of these tests by healthcare authorities and professional societies have further boosted their adoption, contributing to market growth.
Next-generation sequencing (NGS) systems lead the global Non-invasive Prenatal Testing (NIPT) market.
NGS systems provide precise and accurate detection of fetal chromosomal abnormalities by sequencing large amounts of DNA.
They are scalable to meet the increasing demand for NIPT, and their cost-effectiveness makes them preferable to other methods. NGS systems are widely available, improving access for pregnant women. Furthermore, NGS technology enables the development of new NIPT tests that detect a broader range of abnormalities, driving market growth.
In comparison to other techniques like NGS systems, ELISA kits are a generally accessible and affordable choice for NIPT. They are useful for point-of-care testing and smaller laboratories since they are comparatively simple. Trisomy 21 and trisomy 18 are two frequent fetal chromosomal disorders that ELISA tests are highly accurate at identifying. A greater spectrum of abnormalities is detected by ELISA-based NIPT tests, fostering continued market expansion.
The dominance of the high- and average-risk segments in the global Non-invasive Prenatal Testing (NIPT) market is due to their specific needs.
Advancements in technology, increased awareness, and supportive policies have driven the popularity of NIPT. Next-generation sequencing and other advanced techniques have improved accuracy and accessibility. The growing trend of personalized medicine and the desire for informed decision-making during pregnancy have also contributed to the demand for NIPT in both segments.
The demand for NIPT is fueled by the assurance and early knowledge that expectant parents in low-risk pregnancies seek. NIPT has gained popularity due to technological development and public awareness, with the benefits of non-invasiveness, ease, and safety. Adoption of NIPT is further accelerated in the low-risk group by the move toward customized therapy and helpful reimbursement regulations.
Diagnostic centers experience significant growth in the global Non-invasive Prenatal Testing (NIPT) market due to their specialized expertise and equipment.
Collaborations with healthcare providers and hospitals attract more patients. Personalized counseling and support enhance the patient experience. Increasing NIPT demand, awareness, investments, and supportive reimbursement policies drive diagnostic centers' expansion.
The United States has a well-established market for Non-invasive Prenatal Testing (NIPT) tests.
The United States' large healthcare expenditures make NIPT testing widely accessible. The adoption of NIPT testing is made easier by the favorable regulatory environment, which is supported by clear norms and regulations. Leading businesses and academic institutions are pushing innovation in the NIPT sector in the United States, which also has a robust ecosystem for research and development in the sector.
The NIPT market has expanded as a result of the United States' culture of innovation and entrepreneurship, which has also made it more accessible and inexpensive for expectant mothers. Notable businesses involved in the growth and development of the United States NIPT market include Natera, Sequenom, and Ariosa Diagnostics.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 210 |
| Forecast Period | 2022 - 2030 |
| Estimated Market Value (USD) in 2022 | $3.38 Billion |
| Forecasted Market Value (USD) by 2030 | $9.37 Billion |
| Compound Annual Growth Rate | 13.6% |
| Regions Covered | Global |
Company Analysis: Overview, Recent Development, Revenue
- F. Hoffman-La Roche
- Eurofins Scientific
- Illumina
- Natera Inc.
- Revvity Inc.
- Thermo Fisher Scientific
- Quest Diagnostics
- Agilent Technologies
Method - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 4 viewpoints:
- Ultrasound detection
- Biochemical screening tests
- Cell-free DNA in maternal plasma tests
- Others
Technology - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 3 viewpoints:
- ELISA Kits (Enzyme-Linked Immunosorbent Assay Kits)
- Next Generation Sequencing Systems
- Microarray Analysis
Risk Type - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 2 viewpoints:
- High & Average risk
- Low risk
End-User - Global Non-invasive Prenatal Testing (NIPT) Market has been covered from 3 viewpoints:
- Specialty Centers
- Hospitals
- Diagnostic Centers
For more information about this report visit https://www.researchandmarkets.com/r/u770q1
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
