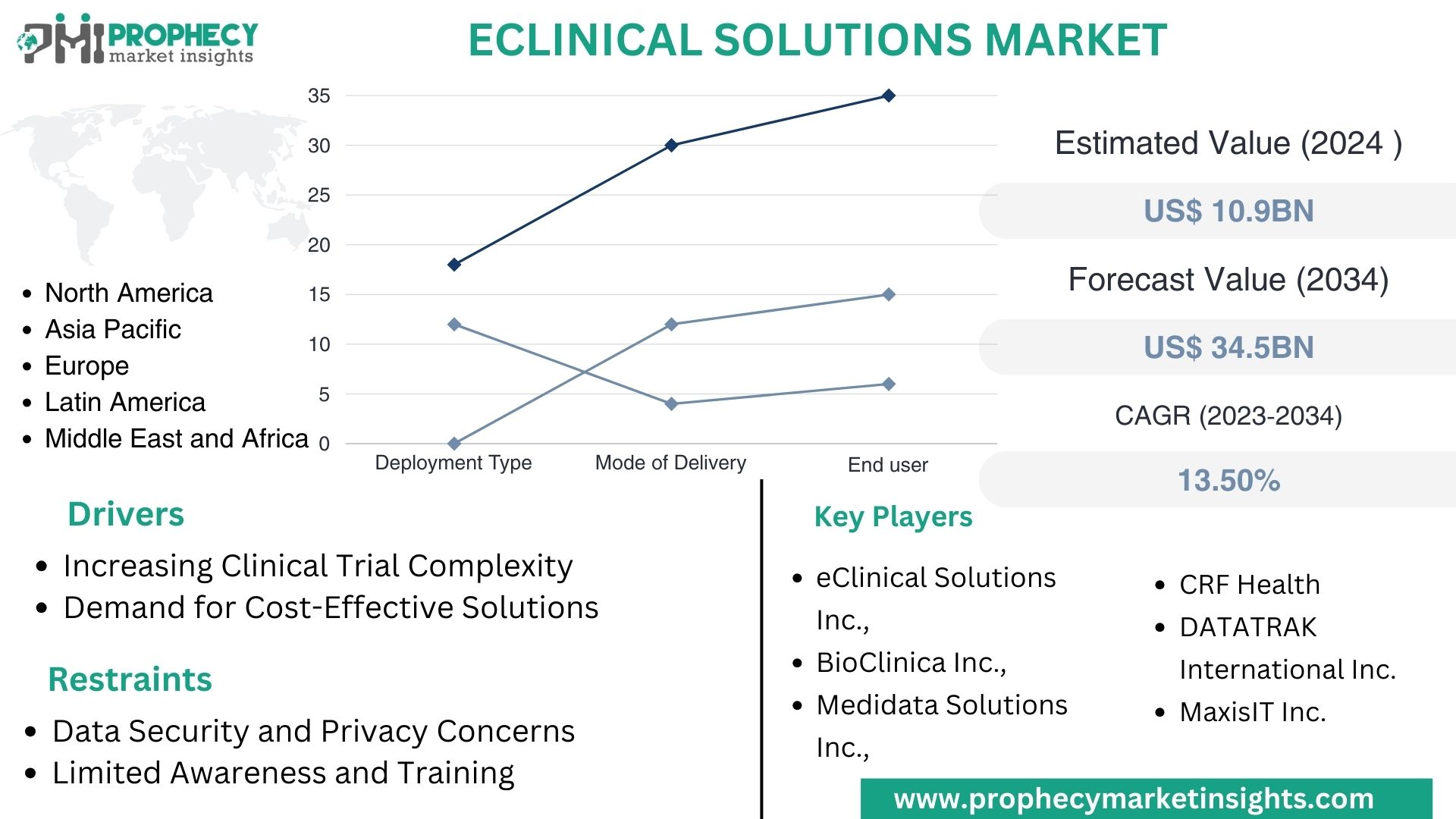
Covina, Feb. 06, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the EClinical Solutions Market size was valued at about USD 10.9 Billion in 2024 and expected to grow at CAGR of 13.5% to extend a value of USD 34.5 Billion by 2034.”
What is eClinical Solutions?
- Market Overview:
eClinical solutions refer to a set of technologies, platforms, and software applications designed to streamline and enhance various aspects of clinical research and trials. These solutions integrate electronic data capture (EDC), clinical trial management systems (CTMS), electronic patient-reported outcomes (ePRO), randomization and trial supply management (RTSM), and other related functionalities to facilitate the conduct of clinical trials.
The primary goal of eClinical solutions is to improve the efficiency, accuracy, and compliance of clinical research processes while reducing the overall time and cost involved in conducting clinical trials. They enable researchers, sponsors, and other stakeholders to collect, manage, analyze, and report clinical trial data in a secure and regulatory-compliant manner.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/31
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Top Leading Players in EClinical Solutions Market:
- eClinical Solutions LLC.
- BioClinica Inc.
- Medidata Solutions Inc.
- Bracket Global LLC.
- DATATRAK International Inc.
- MaxisIT Inc.
- Merge Healthcare Inc.
- OmniComm Systems Inc.
- Oracle Co.
- PAREXEL International Corporation
Market Dynamics:
Driving Factors:
- As clinical trials become more complex due to the demand for novel therapies and personalized medicine, there is a growing need for advanced eClinical solutions to manage the intricacies of trial protocols, data collection, and regulatory compliance.
- The shift from paper-based data collection to electronic data capture systems is driving the adoption of eClinical solutions. EDC systems offer advantages such as improved data accuracy, real-time monitoring, and faster data analysis, thereby enhancing overall trial efficiency.
- Pharmaceutical companies, contract research organizations (CROs), and academic institutions are increasingly seeking cost-effective solutions to streamline clinical trial processes and reduce time-to-market for new drugs and treatments. eClinical solutions offer opportunities for cost savings through improved operational efficiency and reduced data management overheads.
- Innovation in eClinical solutions is being propelled by technological innovations including artificial intelligence (AI), cloud computing, and mobile applications. These technologies improve efficiency and decision-making skills by enabling remote data collection, real-time monitoring, predictive analytics, and automation of clinical trial procedures.
Restrain Factors:
- Data Security and Privacy Concerns
- Integration and Interoperability Issues
- Budgetary Constraints
Emerging Trends and Opportunities in EClinical Solutions Market:
- The adoption of cloud-based eClinical solutions is on the rise due to their scalability, flexibility, and cost-effectiveness. Cloud platforms enable real-time access to trial data from anywhere, streamline collaboration among stakeholders, and support seamless integration with other clinical research systems.
- There is a growing emphasis on incorporating patient-centric approaches into clinical trial design and execution. eClinical solutions that support electronic patient-reported outcomes (ePRO), remote monitoring, and patient engagement tools facilitate active participation, improve patient retention, and enhance overall trial experience.
- AI and ML technologies are being integrated into eClinical solutions to automate data processing, identify patterns and trends in clinical data, and facilitate predictive analytics for risk-based monitoring and decision-making. These capabilities enable faster insights, optimize resource allocation, and improve trial outcomes.
- Particularly in light of the COVID-19 pandemic, virtual trials and decentralised trial methods are becoming more and more popular as alternatives to conventional site-based trials. Greater patient access, a reduction in geographical obstacles, and accelerated trial deadlines are made possible by eClinical technologies that facilitate remote patient recruitment, electronic consent, telemedicine visits, and home-based monitoring.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/31
Challenges of eClinical Solutions Market:
- Maintaining data quality and integrity throughout the clinical trial process is essential for ensuring the validity and reliability of study results. Issues such as data entry errors, missing data, and inconsistencies can compromise the integrity of trial data, leading to inaccurate conclusions and regulatory scrutiny.
- Integrating eClinical solutions with existing electronic health record (EHR) systems and clinical workflows can be challenging. Legacy systems may lack the necessary APIs or interoperability standards, requiring custom development efforts and potentially disrupting established processes.
- Implementing eClinical solutions requires significant financial investment and resources. For smaller organizations or clinical trials with limited budgets, accessing and affording these solutions can be challenging. Additionally, ongoing maintenance costs and training expenses further add to the financial burden.
Detailed Segmentation:
- eClinical Solutions Market, By Deployment Type
- Electronic data capture
- Electronic clinical outcome assessment
- Clinical trial management system
- Clinical analytics platform
- Randomization and trial supply management
- Electronic trial master file
- Others
- eClinical Solutions Market, By Mode of Delivery
- Web/cloud based system
- On premise system
eClinical Solutions Market, By End User
- Pharmaceutical and biotech companies
- Hospitals
- Clinical research organization
- Medical device manufacturers
- Others
eClinical Solution Market, By Region
- North America
-
-
- U.S.
- Canada
-
-
- Europe
-
-
- UK
- France
- Germany
- Russia
- Italy
- Rest of Europe
-
-
- Asia Pacific
-
-
- India
- Japan
- South Korea
- China
- Rest of Asia Pacific
-
-
- Latin America
-
-
- Brazil
- Mexico
- Rest of Latin America
-
-
- Middle East & Africa
-
-
- GCC
- Israel
- South Africa
- Rest of Middle East
-
-
Regional Analysis:
Regional insights highlight the diverse market dynamics, regulatory landscapes, and growth drivers shaping the EClinical Solutions Market across different geographic areas. Understanding regional nuances and market trends is essential for stakeholders to capitalize on emerging opportunities and drive market expansion in the eClinical Solutions sector.
North America dominates the eClinical Solutions market the most as this region leads the eClinical solutions market due to advanced healthcare infrastructure, robust regulatory frameworks, and high research and development expenditure. The region is home to numerous pharmaceutical and biotechnology companies, CROs (Contract Research Organizations), and academic research institutions driving the adoption of eClinical solutions.
Report scope:
| Attribute | Details |
| Market Size 2024 | US$ 10.9 Billion |
| Projected Market Size 2034 | US$ 34.5 Billion |
| CAGR Growth Rate | 13.5% |
| Base year for estimation | 2023 |
| Forecast period | 2024 – 2034 |
| Market representation | Revenue in USD Million & CAGR from 2024 to 2034 |
| Market Segmentation | By Deployment Type - Electronic Data Capture, Electronic Clinical Outcome Assessment, Clinical Data Management System, and Clinical Trial Management System, Clinical Analytics Platform, Randomization and Trial Supply Management, Electronic Trial Master File and Others By Mode of Delivery - Web/Cloud based System and On Premise System By End user - Pharmaceutical And Biotech Companies, Hospitals, Clinical Research Organizations, Medical Device Manufacturers and Others |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key highlights of the EClinical Solutions Market:
- The eClinical solutions market has been experiencing significant growth due to the increasing adoption of electronic data capture (EDC), clinical trial management systems (CTMS), electronic patient reported outcomes (ePRO), electronic clinical outcome assessments (eCOA), and other eClinical technologies. Factors driving market growth include the need for improved efficiency, data quality, and regulatory compliance in clinical trials.
- The eClinical solutions market is witnessing continuous technological advancements, including the integration of artificial intelligence (AI), machine learning (ML), natural language processing (NLP), and blockchain technology.
- The eClinical solutions market is characterized by ongoing consolidation and strategic partnerships among technology vendors, CROs, pharmaceutical companies, and academic research organizations. Mergers, acquisitions, and collaborations aim to enhance product portfolios, expand market reach, and leverage complementary capabilities to address evolving customer needs and market dynamics.
- Globalization of clinical research, patient-centric trends, regulatory requirements, technology improvements, and regulatory restrictions are driving growth and innovation in the eClinical solutions industry.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/31
Explore More Insights:
- Health Information Exchange Market- Trends, Analysis and Forecast till 2034
- Clinical Communication and Collaboration Market– Trends, Analysis and Forecast till 2034
- Population Health Management Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
