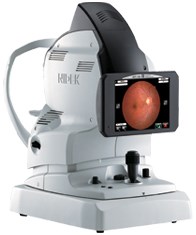
FREMONT, Calif. and JACKSONVILLE, Fla., May 15, 2012 (GLOBE NEWSWIRE) -- NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, announces the FDA 510(k) Clearance for the AFC-330, their most automated fundus camera yet.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=12900
"The AFC-330 represents NIDEK's 3rd generation of automated fundus camera. We are both proud and excited to be leading the way designing and producing fundus cameras that are faster, easier, and more versatile than ever. We anticipate increasing our fundus camera market share with our market expansion with MARCO Ophthalmic."
Motoki Ozawa, President and CEO of NIDEK
"We couldn't be more excited about adding the Nidek AFC-330 automated fundus camera to our full product line of diagnostic technologies. The AFC-330 fits perfectly into Marco's successful model of increasing efficiency with the kind of powerful, easy-to-use, and high-quality instrumentation that our customers have come to expect."
David Marco, President and CEO of MARCO
The AFC-330 makes quantum leaps improving the operator and patient interface, simplicity, automation, and total practice efficiencies. This camera offers an all in one compact design, auto alignment on the X-Y-Z axis, and a wide range of automated features including auto stereo for Glaucoma Management. The lower flash intensity and sound-dampening internal movements mean less retakes and improved patient comfort. No other Non-Mydriatic camera provides both this level of advanced automation and image quality.
While NIDEK will continue to sell to the Ophthalmology market in the United States, MARCO, the leader in Vision Diagnostics, will sell the NIDEK AFC-330 to the Optometry market. This market expansion is to increase the distribution channels and better serve new and existing customers for both companies.
About NIDEK:
Founded in Gamagori, Japan in 1971, NIDEK continues to be a global leader in research and development, design, manufacture and distribution of ophthalmic equipment. The United States subsidiary based in Silicon Valley, California, provides sales and service for ophthalmic lasers, refractive lasers, and many advanced diagnostic devices.
The Nidek Inc. logo is available at http://www.globenewswire.com/newsroom/prs/?pkgid=1006
About MARCO:
Founded in Jacksonville, FL, in 1967, MARCO continues to expand its position as 'The Leader in Vision Diagnostics' with a product line that encompasses classical lane equipment and NIDEK high-tech, automated refractive and retinal instrumentation. MARCO continues to provide unparalleled training and support to its expanding United States customer base.
The photo is also available at Newscom, www.newscom.com, and via AP Photo Express.