BETHESDA, Md., Aug. 13, 2012 (GLOBE NEWSWIRE) -- Spherix Incorporated (Nadsaq:SPEX) – an innovator in biotechnology for therapy in diabetes, metabolic syndrome and atherosclerosis, and provider of technical and regulatory consulting services to food, supplement, biotechnology and pharmaceutical companies – today announced that its investigational drug candidate SPX106T (a combination of SPX106 and D-tagatose) significantly reduced the growth rate of abdominal aortic aneurysms (AAAs) in two different strains of mice prone to hyperlipidemia.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=14020
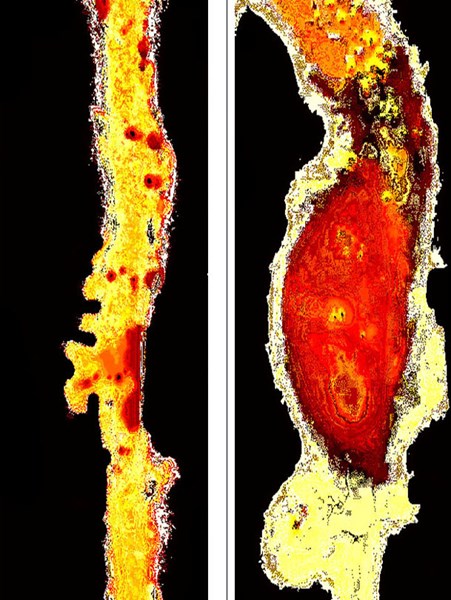
In mice, AAAs can be provoked by hyperlipidemia and elevated blood pressure. In this study apolipoprotein E-deficient (ApoE -/-) mice were given a Western diet to promote hyperlipidemia and treated with SPX106T for four weeks before implantation of osmotic minipumps containing angiotensin II (angII); infusion of angiotensin was continued for an additional two weeks during the treatment. Statistical analysis showed that the growth curves of the diameters from aortas in the untreated ApoE -/- mice were greater than the growth curves in the mice treated with SPX106T (p<0.05). Greater growth indicates dilation of the aneurysm, with a concomitant increase in the risk of rupture and sudden death. The study was conducted after a pilot study in low density-lipoprotein receptor-deficient (LDLR-/-) mice given a Western diet, treated with SPX106T and infused with ang II, showed a treatment-related reduction in aortic diameter growth curves and atherosclerosis1. False-color images of the abdominal aortas from an LDLR-/- mouse treated with SPX106T that developed an aneurysm, and an LDLR-/- mouse that received no drug treatment and developed an aneurysm are shown in Figure 1.
Although the causes of AAAs in humans are unclear, risk factors include age, gender, genetics, smoking, atherosclerosis, hyperlipidemia, high blood pressure and cerebrovascular disease. Spherix's research into the mechanisms of action of SPX106T suggests that its pharmacologic activity may be a result of lowering blood lipids. Others have reported that a metabolite of SPX106 (a component of SPX106T) lowers matrix metalloproteinase-9 (MMP-9). MMP-9 is a proteolytic enzyme produced by vessel wall infiltrating macrophages that contributes to wall weakening by degrading structural components, including collagen. Tissue sections from the aortas of SPX106T treated LDLR-/- mice had more collagen than untreated mice, corroborating MMP-9 reduction as a possible mechanism by which SPX106T preserves collagen and reduces the rate of aneurysm growth.
"These study results further our goal of expanding the application of SPX106T to multiple indications where dyslipidemia has been suggested as a primary risk factor," said Dr. Claire Kruger, Spherix's CEO, "Spherix is dedicated to continuing the innovative research that will deepen our pharmaceutical pipeline, and treatment of AAAs represents a potential new use for SPX106T. We are now exploring grant applications, possibly in conjunction with a pharmaceutical partner, as part of our plan to further develop SPX106T for this indication."
About Spherix
Spherix Incorporated was launched in 1967 as a scientific research company under the name Biospherics Research. The Company now leverages its scientific and technical expertise and experience through its two subsidiaries – Biospherics Incorporated and Spherix Consulting, Inc. Biospherics is dedicated to developing and licensing/marketing proprietary therapeutic products for treatment of diabetes, metabolic syndrome and atherosclerosis. Biospherics is exploring new drugs and combinations for treatment of high triglycerides, a risk factor for atherosclerosis, myocardial infarction, and stroke. Spherix's Consulting subsidiary provides scientific and strategic support for suppliers, manufacturers, distributors and retailers of conventional foods, biotechnology-derived foods, medical foods, infant formulas, food ingredients, dietary supplements, food contact substances, pharmaceuticals, medical devices, consumer products and industrial chemicals and pesticides. For more information, please visit www.spherix.com.
The Spherix Incorporated logo is available at http://www.globenewswire.com/newsroom/prs/?pkgid=14019
Forward-Looking Statements
This release contains forward-looking statements which are made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such statements in this release, including statements relating to planned clinical study design, regulatory and business strategies, plans and objectives of management and growth opportunities for existing or proposed products, constitute forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those anticipated by the forward-looking statements. The risks and uncertainties include, without limitation, risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured, we may lack financial resources to complete development of our products, the FDA may interpret the results of studies differently than us, competing products may be more successful, demand for new pharmaceutical products may decrease, the biopharmaceutical industry may experience negative market trends, our continuing efforts to develop products may be unsuccessful, our common stock could be delisted from the Nasdaq Capital Market, and other risks and challenges detailed in our filings with the U.S. Securities and Exchange Commission. Readers are cautioned not to place undue reliance on any forward-looking statements which speak only as of the date of this release. We undertake no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this release or to reflect the occurrence of unanticipated events.
1In these mice, linear discriminant analysis of the growth curves assigned the mice to SPX106T-treated or Control groups with 100% sensitivity and 100% specificity. Validation of these results using the method of Maitra et al. indicated a sensitivity of 75% and a specificity of 75%.
The photo is also available at Newscom, www.newscom.com, and via AP PhotoExpress.