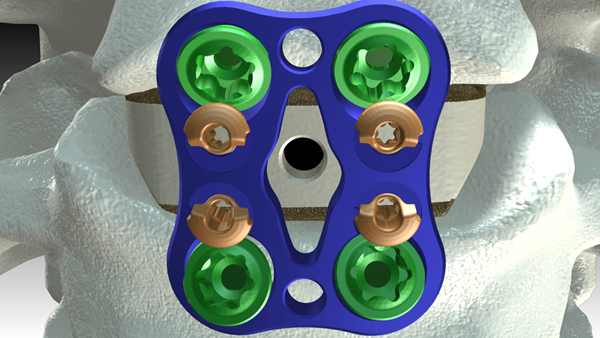
FLORENCE, S.C., Sept. 17, 2015 (GLOBE NEWSWIRE) -- DeGen Medical has received clearance from the FDA for its first cervical spine implant, Hyper-C™ Anterior Cervical Plate System. The Hyper-C™ system is an anterior intervertebral fixation device that is designed to aid in stabilizing the cervical spine.
"Clinical research has shown that greater than 5mm distance (of the cervical plate) from adjacent discs equates to less adjacent level degeneration post-operatively. The small over-hang of Hyper-C™ plate is an evidence based approach to attain this outcome which may be extremely beneficial for patients in decreasing adjacent level disease*." - Dr. Rakesh Chokshi.
"The super-short Hyper-C™ plate allows for hyper-angulation of bone screws in convergent or divergent orientation. This allows more intraoperative flexibility for primary and revision surgery with existing hardware" – Dr. W. S. (Bill) Edwards Jr.
The Hyper-C™ Anterior Cervical Plate System offers:
- Plates in levels 1 thru 5 in addition to multiple lengths
- Bone screws in 4.0mm, 4.25mm, and 4.6mm diameters
- Fixed or variable bone screw choices as well as self-drilling or self-tapping option
The Hyper-C™ will be available in Q4 of 2015.
*This statement is based on references from clinical articles and has not been confirmed with clinical trials.
About DeGen Medical, Inc.:
DeGen Medical, Inc. is a medical-device development company dedicated to providing surgeons with innovative products engineered to improve quality of life for patients with complex spinal disorders. World-class implants, coupled with intuitively-designed instrumentation, provide a complete package to promote superior surgical outcomes. Our passion to advance spine-care solutions is driven by clinical insights, sound research, and science-based design. DeGen Medical maintains the highest quality standards to provide reliable products and safeguard patient health.
A Photo accompanying this release is available at:
http://www.globenewswire.com/newsroom/prs/?pkgid=36211