FREMONT, CA--(Marketwired - Feb 12, 2016) - Optovue, the global leader in the development and commercialization of optical coherence tomography angiography (OCTA), today announced FDA clearance and immediate U.S. commercial availability of the AngioVue™ Imaging System, a groundbreaking technology that provides a non-invasive way to visualize abnormal blood vessels in the retina to help physicians determine an appropriate course of treatment.
"We are thrilled to announce FDA clearance of our AngioVue System, which will bring significant benefits of our innovative, non-invasive retinal imaging to patients in the US suffering from retinal diseases that lead to progressive blindness," said Jay Wei, founder and chief executive officer at Optovue. "Since we first introduced this technology to markets outside the US 14 months ago, the technology is in daily clinical use at over 525 clinical sites where it provides a more patient-friendly approach to diseases of the retina that lead to progressive blindness."
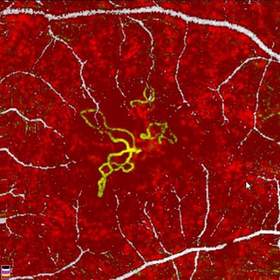
The AngioVue System, with its proprietary technology, provides physicians with a non-invasive, dyeless technique for quickly visualizing the presence or absence of flow in the blood vessels. This enables the assessment of new information from the microvasculature and perfusion in ocular diseases with extraordinary detail.
Optovue is the first company to develop and commercialize this pioneering OCTA technology. Utilizing light rays to form detailed three-dimensional images of the retina, physicians are able to quickly visualize the blood vessels. In less than three seconds, the AngioVue System acquires a single image that complements the current angiography imaging standard, fluorescein angiography (FA), but with a number of advantages. Unlike FA, the AngioVue System does not require the use of dye injections, which can often obscure the target anatomy and can lead to side effects and other complications associated with dye-based, invasive procedures.
About Optovue
Optovue, Inc., a privately-held medical device company founded in 2003 and based in Fremont, Calif., is dedicated to the development and commercialization of high-speed optical coherence technology used to facilitate the diagnosis and management of eye diseases, many of which may lead to permanent blindness. For more information, visit www.optovue.com.
Contact Information:
Media Contact:
Amy Cook
925.200.2125