EFA also secures US$6.5 Million in funding, backed by existing investors and new partners
Tel Aviv, Israel, 10 April 2024 - EFA Technologies, pioneers in portable blood labs, today announces the start of its operations in Brazil, following approval last October of its leading portable blood lab device, RevDx, by Anvisa, the Brazilian health regulatory authority. The company also secured US$6.5 million in funding, boosting EFA’s mission to further improve healthcare worldwide.
RevDx, EFA’s flagship product, is a sophisticated portable diagnostic device that leverages advanced technology to provide accurate and rapid medical testing locally. Its capabilities include identifying pathogens, analyzing biomarkers and aiding in disease diagnosis. Thereby revolutionizing the field of on-site diagnostics with its efficiency and precision.
RevDx marks a significant breakthrough in medical diagnostics. It is portable, affordable and user-friendly, leveraging microscopy and AI to deliver instant results. This not only reduces costs and saves time, but also improves the doctor-patient relationship. With the capability to perform a full CBC count from just a single drop of blood, the device streamlines diagnostics and equips healthcare professionals with actionable insights.
Yoel Ezra, CEO of EFA Technologies:
"We’re thrilled to witness the impact of RevDx's on healthcare. Our mission to make Medtech accessible to everybody, starting with a complete blood count test (CBC), is coming to fruition. We’re dedicated to further expanding our reach for the benefit of patients and caregivers worldwide. Entering the vast and promising Brazilian market is a testament to that."
Backed by a distinguished medical board that includes Prof. Aaron Ciechanover, a 2004 Nobel Prize Laureate, EFA Technologies is poised to revolutionize healthcare on a global scale. Prof. Ciechanover's endorsement underscores the significance of RevDx's medical innovation.
"The potential of this 'little' device is enormous," says Prof. Ciechanover. "It opens doors to myriad clinical applications, simplifying complex processes and transforming patient care."
EFA Technologies has obtained CE certification and enters the Brazilian market with ANVISA certification for RevDx. Meanwhile, EFA is actively exploring new markets for further expansion.
Amir Lubshevsky, EFA’s Chief Medical Officer, adds:
"We're thrilled by the initial positive response from the Brazilian market. It's an exciting time for EFA Technologies as we enter this promising market. We're eager to solidify our presence and welcome further international interest in our innovative solutions."
New financing round
In addition, EFA has secured another US$6.5 million in growth capital. The current round was led by existing investors and new partners such as E-Health Ventures, Venturing Tech, ARC impact ventures and the Israeli Innovation Authority. This capital injection will accelerate product development, expand market reach and foster innovation in healthcare diagnostics.
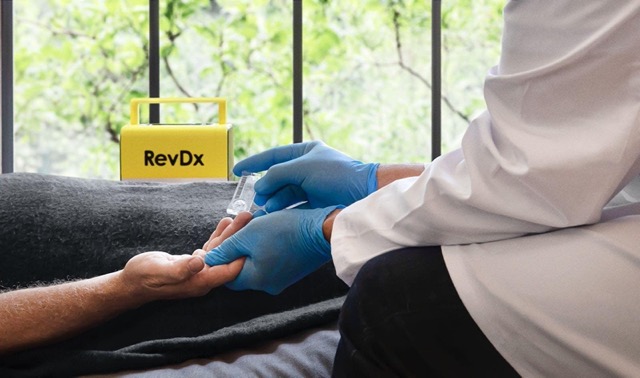
RevDx, is a sophisticated portable diagnostic device that leverages advanced technology to provide accurate and rapid medical testing locally. Its capabilities include identifying pathogens, analyzing biomarkers and aiding in disease diagnosis. Thereby revolutionizing the field of on-site diagnostics with its efficiency and precision.
About EFA Technologies
EFA (Engineering for All) Technologies, based in Caesarea, Israel, was established in 2016 by a team with combined experience in medical sciences, optical and bioengineering, and a vision to bring access to proper diagnostic and healthcare to all.
EFA’s business strategy is to develop its RevDx solution as a platform that will enable the creation of an ecosystem that can develop more diagnostic applications over time. RevDx innovation fits this approach in both its design and capabilities. EFA Technologies packages multi-channel capabilities into the device, starting with automatic microscopy and bio-sensing readers. The digital images and data generated from the system are the basis for further image-processed diagnostics and algorithms.
As EFA Technologies continues its journey of innovation and expansion, its commitment to improving healthcare accessibility and effectiveness remains steadfast. With RevDx at the forefront and underpinned by robust financial backing, EFA is primed to redefine medical diagnostics and enhance patient outcomes worldwide.
For more info please visit: efa-technologies.com, or find us on LinkedIn
Our legal advisors: Naschitz Brandes Amir Ltd. www.nblow.com
Contact for media:
EFA Technologies
Amir Lubashevsky
Chief Marketing Officer
T:+972-544-506-334
E: amirl@efa-technologies.com
LifeSpring Life Sciences Communication, Amsterdam, the Netherlands
Leon Melens
T: +31 6 538 16427
E: lmelens@lifespring.bio
Attachment
