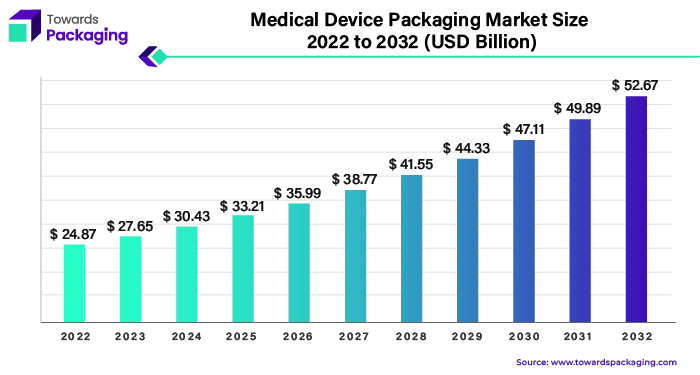
Ottawa, April 24, 2024 (GLOBE NEWSWIRE) -- The global medical device packaging market size was valued at USD 27.65 billion in 2023 and is predicted to surpass around USD 49.89 billion by 2031, a study published by Towards Packaging a sister firm of Precedence Research.
Report Highlights: Important Revelations
- North America central hub for the medical device packaging industry.
- Rising market prospects in medical device packaging across the Asia-Pacific region.
- Essential role of plastic in packaging for medical devices.
- Medical device packaging pouches: ensuring precise device protection.
- Global momentum in sterile medical device packaging.
- Direct distribution channel for medical device packaging solutions.
- Critical role of packaging in monitoring and diagnostic equipment.
- Importance of labels in the medical device sector.
For the short version of this report @ https://www.towardspackaging.com/personalized-scope/5149
Medical device packaging is made up of the materials and processes used to encapsulate, protect, and display medical equipment. It is an essential component of the device's entire design and development process. This encapsulation encompasses not only the physical containers and wraps, but also the meticulous planning, manufacturing, and labelling procedures that maintain the devices' integrity, sterility, and usability during storage, transit, and distribution. Adopting medical device packaging standards allows for the adoption of effective packaging solutions early in the product development process. This proactive method not only decreases stress, but it also encourages the creation of a more streamlined and impactful product, eliminating unforeseen costs and mitigating risks. Medical packaging serves as vital for the safe and efficient delivery of drugs, equipment, and other medical supplies. It not only protects the goods during transportation and storage, but it also serves as a barrier to contamination and deterioration, especially in high-traffic areas like the emergency room.
A constant focus on broad objectives is critical throughout the design process. The intricacies of medical device package design have a substantial impact on a variety of factors, including transportation logistics, user contact, and sterilisation procedures. Anticipating post-usage scenarios is also essential for ensuring flawless performance once the device is in the hands of end users. It is challenging to stay current with the most recent global standards for medical device packaging.
If you have any questions, please feel free to contact us at sales@towardspackaging.com
Manufacturers must closely follow these standards to assure product safety, efficacy, and compliance with end-user specifications. Compliance involves a wide range of requirements, including stringent quality control systems, comprehensive labelling processes, and sophisticated traceability mechanisms. To preserve compliance and market relevance in an ever-changing regulatory context, regular monitoring and agility are essential. Proactive cooperation with regulatory agencies and industry consortia can provide early warning of upcoming changes, allowing manufacturers to alter their packaging strategy and increase their market position.
Medical device packaging is an essential component of the entire product lifecycle, necessitating meticulous attention to detail and strict adherence to evolving regulatory regulations. Manufacturers can handle the complexity of packaging design, safeguard product integrity, and boost consumer trust in their offerings by including efficient packaging solutions early in the design process and being informed about regulatory changes.
Customize this study as per your requirement @ https://www.towardspackaging.com/customization/5149
For Instance,
- In January 2024, Sanner Group, a global leader in healthcare packaging and medical device Contract Development and Manufacturing Organisation (CDMO), has acquired Springboard, a medical device design and development expert for regulated markets.
Medical Device Packaging Market Trends
- Medical device packaging is crucial for protecting devices from infection, damage, and tampering throughout storage, shipping, and handling.
- Considering medical equipment is so variable, customised packaging solutions are required to meet individual product criteria and application needs.
- Packaging technology advancements have resulted in the creation of novel packaging solutions for medical devices.
- Sustainability is becoming more vital in the medical device packaging market as people become more conscious of environmental challenges and sustainability concerns.
North America Hub of Medical Device Packaging Business
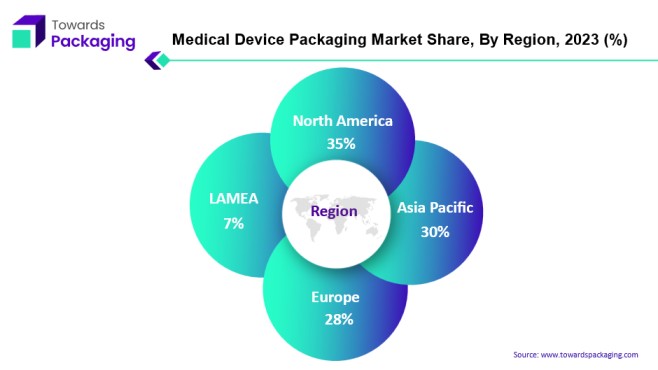
North America serves as the main epicenter for the medical device packaging business. In particular, the United States is notable since in 2022, its imports of medical devices climbed dramatically, from 48% to 710,245 crore. However, there is also a waste disposal issue brought on by the growing amount of medical equipment. The amount of waste that US healthcare facilities have to manage is staggering—nearly 14,000 Tonnes each day. Plastic products and packaging account for between twenty and twenty-five percent of this waste. Furthermore, a noteworthy 85% of the waste generated in hospitals is categorised as non-hazardous, indicating that it is neither contaminated nor comes into touch with patients.
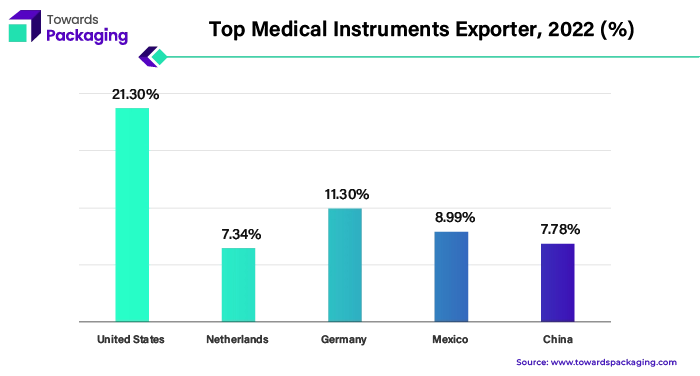
When it comes to the export of medical instruments, the US has become a significant player in the global market. In 2022, the United States topped the export rankings, closely followed by Germany, Mexico, China, and the Netherlands. However, the United States remained the leading importer of medical equipment, with the Netherlands, Germany, China, and Japan following closely behind. These export-import dynamics demonstrate how important the U.S. market is to the global medical device industry and how much of an impact it has on the medical device packaging industry.
For Instance,
- In January 2024, Contract manufacturer Arterex acquired Micromold, a business that uses plastic injection moulding to create precision, customised micromolded parts for the medical sector.
Expanding Market Opportunities in Medical Device Packaging in the Asia-Pacific
The market for medical device packaging is expanding significantly in the Asia-Pacific area, especially in China. Due to advantageous import charges that range from 0% to 10%—the majority of which fall within the 7.5% category—and the one item that is subject to a 25% tax, China now holds a large market share. To help firms survive and promote fruitful partnerships with academic institutions for innovation in process and product development, tariffs are being called for to be lowered to a nominal 15%.
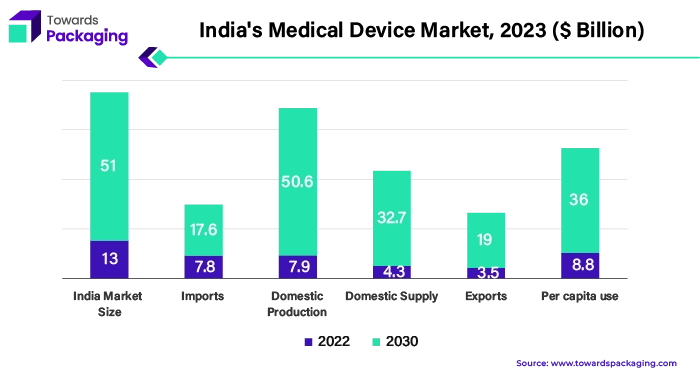
Medical device production and consumption have seen a noticeable upsurge in the Asia-Pacific region, which includes China, India, Japan, South Korea, and Australia. Medical technology breakthroughs, rising healthcare costs, and population expansion are the main causes of this spike. The yearly need for medical equipment is $ 13 billion, of which 65% is met by imports, despite India producing medical devices valued at about $7.68 billion, of which $3.5 billion are exported and $4.3 billion are utilized locally. Only 35 per cent of market demands are met by domestic enterprises, highlighting the need for improvement to lessen reliance on imports and strengthen India's position in the medical device sector.
The Asia-Pacific region has experienced an increase in demand for innovative and exceptional medical device packaging solutions due to factors like a rising number of chronic diseases, ageing populations, and a greater emphasis on healthcare quality and safety. Businesses in the medical device packaging industry have a lot of potential in the Asia-Pacific area. In utilizing the rapidly growing healthcare sector in the region and adapting to the changing demands of customers and legal specifications, businesses can forge a solid foundation and propel expansion in an ever-changing market sector.
For Instance,
- In September 2023, India's medical device business has expanded at a constant rate of 14%-15% per year for the most of the last decade, although it still accounts for only 1.5% of the total worldwide market.
Medical Device Packaging Market, DRO
Demand:
- Demand for Maintaining sterility is essential in medical device packaging to avoid microbiological infection and assure patient safety.
Restraint:
- The global nature of the medical device industry, combined with complicated supply chains, complicates medical device packaging logistics, which include sourcing, production, sterilisation, transportation, and distribution.
Opportunity:
- The increased emphasis on sustainability and environmental responsibility creates an opportunity for the development of environmentally friendly medical device packaging.
Indispensable Role of Plastic in Medical Device Packaging
Plastic is essential for medical device packaging because of its versatility, durability, and compliance with sterilization techniques. Polyethylene, polypropylene, and polycarbonate are common materials used in medical packaging, achieving a mix of attributes necessary for device integrity and safety. These packaging are critical for the safe distribution and storage of medicinal items, emphasizing the necessity of contamination-resistant designs, particularly in healthcare settings where patient safety is important. PP is a prominent material used in medical device packaging. It is an extremely durable substance that can withstand impact, moisture, and chemicals. PP is also a great moisture barrier, making it perfect for packing medical items that need to be kept sterile.
Organizations such as Sterile Aware and Van der Stahl Scientific are working to improve sterile packaging systems through novel methodologies such as medical device pouch testing. Their ISO 17025 empirical testing facility and co-developed testing technologies address infection concerns associated with packaging problems.
Plastic acts as an excellent barrier against contamination, moisture, and environmental conditions that may jeopardies device sterility and performance. Its flexibility to be molded into a variety of forms and sizes enables customised packaging solutions. Despite environmental worries, plastic has substantial benefits for the industry.
The lightweight nature of plastic packaging allows for more cost-effective shipping and distribution of medical devices while also decreasing the total environmental footprint. Furthermore, plastics can tolerate sterilisation procedures such as gamma radiation, ethylene oxide gas, and steam autoclaving, guaranteeing that devices are sterile until use. In conclusion, plastics are critical for maintaining the quality and performance of medical devices throughout their existence.
For Instance,
- In January 2024, Arterex, a contract manufacturer, announced the acquisition of Micromold, Inc. Arterex, a contract manufacturer, has acquired Micromold, a company that manufactures unique quality micromoulded parts for the medical market using plastic injection moulding.
Medical Device Packing Pouches Safeguarding Medical Devices with Precision
Medical device packing pouches are specialised containers made to enclose and safeguard particular medical devices while they are being distributed, stored, and transported. The materials used to make these pouches are usually laminates or plastic films, which are selected for their compatibility with sterilisation procedures, durability, and barrier qualities. Pouches can be manufactured of several types of plastic, such as polyethylene and polypropylene, and are commonly used for medical devices and equipment. One way to preserve single-use items and do away with the need to sterilise them beforehand is to seal plastic bags.
Maintaining product integrity and preventing contamination are made possible by the design of medical device packing pouches. Pouches must successfully protect electronics from outside elements like dust, moisture, and germs so that they stay sterile until needed., Pouches ought to be effortlessly opened and sealed to enable convenient access to the contained gadget while maintaining its sterility. To fit a range of device types, from tiny instruments to larger equipment, medical device packing pouches are available in multiple sizes and designs. Patients and medical professionals might be reassured by some pouches that have signs to verify sterilisation procedures.
Medical device packing pouches are critical to ensure the safety, efficacy, and quality of medical devices throughout their life cycle. Emphasising the value of accuracy and meticulous attention to detail in their creation, their design and material selection are meticulously adjusted to match the particular requirements of each device.
For Instance,
- In March 2023, The Sterimed Group has successfully acquired Granton Medical, a UK-based producer of sterilisation pouches and supplier of packing services to the medical device market, through its UK subsidiary Westfield Medical.
Global Acceleration to Sterile Medical Device Packaging
Medical device packaging serves as a critical safeguard against contamination, particularly for sterile products intended for invasive, implantable, or single-use applications that involve contact with human tissues or fluids. This packaging acts as a barrier between the device and its surroundings, shielding it from potential contaminants during storage, transportation, and handling. Sterile medical device packaging employs materials such as foil bags, Tyvek pouches, and rigid containers, selected for their low permeability to water vapor and air. This characteristic is essential in preventing the ingress of microorganisms that could compromise the sterility of the enclosed device.
Before sealing, sterilization techniques such as gamma irradiation or ethylene oxide gas are often employed to eradicate any microbial contaminants present on the device or within the packaging itself. These measures are crucial in reducing the risk of infections, as hospital-acquired infections contribute significantly to global mortality rates, with millions of deaths reported annually due to such infections. Due to this factor market for sterile medical device packaging is estimated to increase at high rate.
The primary types of sterile packaging commonly utilized in medical devices include trays made of metal or plastic and pouches constructed from paper or plastics. Each type is tailored to the specific requirements of the device it encases, ensuring optimal protection and sterility throughout the product's lifecycle. By employing stringent sterilization and packaging protocols, medical device manufacturers aim to mitigate the risk of infections and uphold the safety and efficacy of their products for patients and healthcare providers alike.
For Instance,
- In November 2023, the founders of SteriPack Ireland Limited ("SteriPack Ireland"), Garry Moore, and Sterimed Infection Control have announced their decision to pool their business operations in the field of medical packaging. It meets all the requirements for both single-use and reusable sterile medical equipment packaging.
Direct Distribution Channel for Medical Device Packaging
Manufacturers who offer their packaging materials directly to end users or consumers, eschewing middlemen like distributors or wholesalers, are contributing to the direct distribution channel for medical device packaging. The distribution, marketing, and sales of the packaging solutions are directly under the makers' control in this channel. For both producers and buyers, this distribution strategy has a number of benefits. It gives producers more control regarding terms of branding, price, and customer relations. McKesson Medical-Surgical, the largest wholesale distributor of medical-surgical supplies and equipment in the United States, generated USD 231.1 billion in revenue during the past year.
They offer specialised assistance and service and can modify their packaging solutions to suit the needs of individual clients. Manufacturers can also benefit from increased value chain capture and possible profit margin expansion through direct distribution. Customers can save money by purchasing directly from manufacturers, as there are no markups from intermediaries. It also gives you direct access to the manufacturer's expertise and assistance, which means you get better service and are more responsive to your needs.
The direct distribution channel for medical device packaging has expanded significantly in recent years. This expansion can be ascribed to a number of factors, including increased need for specialised packaging solutions as the medical device industry expands, advancements in packaging technology, and the trend towards customisation and personalised service.
- In February 2024, Riverside Medical Packaging, a UK-based contract packaging provider for medical devices, has announced the demerger of its Shawpak business unit.
Importance of Packaging for Monitoring and Diagnostic Equipment
End-user monitoring and diagnostic equipment in medical device packaging includes specialised devices that monitor and diagnose patients' health status or physiological characteristics. These devices are critical instruments in healthcare settings, allowing experts to collect precise data for diagnosis, treatment, and monitoring of patients' ailments.
Medical device packaging for monitoring and diagnostic equipment fulfils multiple vital functions. It preserves these devices' sensitive components throughout storage, transportation, and handling, ensuring they are functional and accurate when deployed. Packaging must ensure the sterility of any components that come into touch with patients or body fluids in order to avoid contamination and infection.
Regulatory compliance is a key feature of medical device packaging for monitoring and diagnostic equipment. To guarantee that the devices are approved and marketable, manufacturers must ensure that packaging materials and methods meet regulatory criteria for safety, sterility, and labelling.
For Instance,
- In January 2024, A second manufacturing facility and new production location in Bensheim, Germany, as well as the company's headquarters, position the Sanner Group for future expansion.
Significance of Labels in the Medical Device Industry
Labels are an essential means of communication between manufacturers and consumers in a number of industries, such as pharmaceuticals, food goods, and medical equipment. Labels are extremely important in the medical device industry when it comes to products meant for human use. They offer vital details including how to use them, who the intended users are, any risks, and safety measures.
It is essential for medical device manufacturers to make sure that labels are readable and undamaged for the duration of the product. For labels to properly convey vital information to end users, they must endure handling, storage, distribution, and use. Patient safety may be jeopardised by a compliance label that separates or becomes unreadable during storage or transit. Thus, in order to preserve regulatory compliance and guarantee the safe and proper use of medical equipment, businesses must place a high priority on the longevity and adherence of labels.
For Instance,
- In February 2024, Coveris, a European supplier of sustainable packaging solutions, has made an investment in its label business by purchasing S&K LABEL, a company situated in the Czech Republic.
Key Players and Competitive Dynamics in the Medical Device Packaging Market
The competitive landscape of the medical device packaging market is dominated by established industry giants such as Berry Global Inc., Amcor Limited, Mitsubishi Chemical Holdings Corporation, 3M COMPANY, CCL Industries Inc., Constantia Flexibles, Klöckner Pentaplast Group, Sonoco Products Company, Dupont De Nemours Oliver-Tolas, NIPRO, and Texchem-pack. These giants compete with upstart direct-to-consumer firms that use digital platforms to gain market share. Key competitive characteristics include product innovation, sustainable practices, and the ability to respond to changing consumer tastes.
Berry Global prioritises innovation and customisation to satisfy the changing needs of the medical device sector. The company invests substantially in R&D to provide cutting-edge packaging solutions that prioritise safety, sterility, and usability. Berry Global also prioritises collaboration with medical device makers to understand their individual needs and customise packaging solutions accordingly.
For Instance,
- In September 2023, Berry Global Group Inc. (BERY) disclosed that it has begun a process to assess strategic options for its health, hygiene, and specialties sector.
Amcor has adopted a global expansion plan to increase its footprint in the medical device packaging market. The company uses its large global network and manufacturing skills to provide a wide range of packaging solutions targeted to specific locations and customer segments.
For Instance,
- In January 2023, Amcor, a global packaging company, has reached an agreement to buy MDK, a medical device packaging company based in China. MDK, a Shanghai-based company, provides paper-based packaging and coating services to medical device manufacturers in China.
Browse More Insights of Towards Packaging:
- The global alcoholic beverage packaging market size expected to increase from USD 66.68 billion in 2022 to obtain a projected USD 116.63 billion by 2032 at a growing CAGR of 5.8% CAGR between 2023 and 2032.
- The global form-fill-seal packaging market size anticipated to rise from USD 8.97 billion in 2022 to attain a calculated USD 14.96 billion by 2032 at a growing CAGR of 5.3% CAGR between 2023 and 2032.
- The global pet care packaging market size forecasted to expand from USD 11.2 billion in 2022 to reaching an estimated USD 17.52 billion by 2032 at a growing CAGR of 4.6% CAGR between 2023 and 2032.
- The worldwide refillable packaging market size forecasted to expand from USD 41.50 billion in 2022 to reaching an estimated USD 61.72 billion by 2032 at a growing CAGR of 4.1% CAGR between 2023 and 2032.
- The global dairy product packaging market size forecasted to expand from USD 31.45 billion in 2022 to achieve an approximation USD 51.96 billion by 2032 at a growing CAGR of 5.2% CAGR between 2023 and 2032.
- The global hermetic packaging market size calculated to go up from USD 4.31 billion in 2022 to accomplish a supposed USD 7.22 billion by 2032 at a growing CAGR of 5.3% CAGR between 2023 and 2032.
- The global consumer packaged goods (CPG) market size presumed to grow from USD 2,132.1 billion in 2022 to realize an expected USD 3,171.11 billion by 2032 at a growing CAGR of 4.1% CAGR between 2023 and 2032.
- The global thin wall packaging market size speculated to escalate from USD 41.48 billion in 2022 to fulfill a guesstimated USD 76.77 billion by 2032 at a growing CAGR of 6.4% CAGR between 2023 and 2032.
- The global dunnage packaging market size envisioned to advance from USD 3.8 billion in 2022 to reach a conjectured USD 6.52 billion by 2032 at a growing CAGR of 5.6% CAGR between 2023 and 2032.
- The global stick packaging market size is envisaged to surge from USD 325.21 million in 2022 to acquire USD 547.63 million by 2032, at a growing 5.4% CAGR between 2023 and 2032.
Medical Device Packaging Market Player
Medical device packaging leading market players are Berry Global Inc., Amcor Limited, Mitsubishi Chemical Holdings Corporation, 3M COMPANY, CCL Industries Inc., Constantia Flexibles, Klöckner Pentaplast Group, Sonoco Products Company, Dupont De Nemours Oliver-Tolas, NIPRO, and Texchem-pack.
Market Segments
By Material
- Plastic
- Glass
- Paper & Paperboard
- Aluminium
- Metal
- Others
By Product Type
- Pouches
- Bags
- Trays
- Containers
- Boxes
- Blisters
- Clamshell Packs
- Others
By Application
- Sterile Packaging
- Non-Sterile Packaging
By Distribution Channel
- Direct
- Retail
By End User
- Monitoring & Diagnostic Equipment
- Disposable Consumables
- Therapeutic Equipment
By Accessories
- Labels
- Lidding
- Others
By Region
- North America
- Asia Pacific
- Europe
- LAMEA
Own your copy of our reach study and stay informed: https://www.towardspackaging.com/price/5149
Explore the statistics and insights concerning the packaging industry and its segmentation: Subscribe to Annual Membership
If you have any questions, please feel free to contact us at sales@towardspackaging.com
About Us
Towards Packaging is a leading global consulting firm specializing in providing comprehensive and strategic research solutions. With a highly skilled and experienced consultant team, we offer a wide range of services designed to empower businesses with valuable insights and actionable recommendations. We stay abreast of the latest industry trends and emerging markets to provide our clients with an unrivalled understanding of their respective sectors. We adhere to rigorous research methodologies, combining primary and secondary research to ensure accuracy and reliability. Our data-driven approach and advanced analytics enable us to unearth actionable insights and make informed recommendations. We are committed to delivering excellence in all our endeavours. Our dedication to quality and continuous improvement has earned us the trust and loyalty of clients worldwide.
Browse our Brand-New Journals:
https://www.towardshealthcare.com
http://www.towardsautomotive.com
Web: https://www.precedenceresearch.com
For Latest Update Follow Us: https://www.linkedin.com/company/towards-packaging
Get Our Freshly Printed Chronicle: https://www.packagingwebwire.com
