Dublin, Nov. 14, 2025 (GLOBE NEWSWIRE) -- The "India Immunoglobin Market Outlook 2025-2033" has been added to ResearchAndMarkets.com's offering.
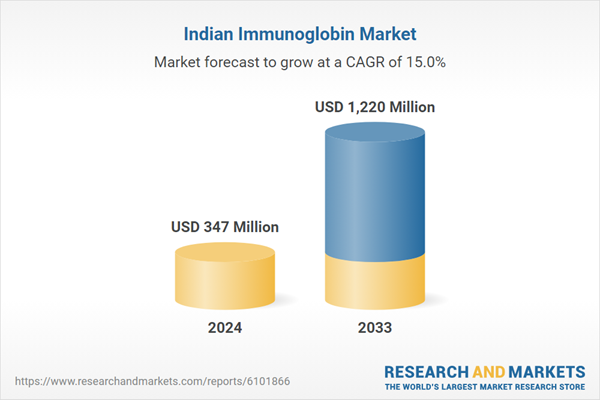
India's Immunoglobulin Market is expected to reach US$ 1.22 billion by 2033 from US$ 347 million in 2024, with a CAGR of 15% from 2025 to 2033. The rising prevalence of immunodeficiency disorders, plasma-derived therapies, government favors, growing health awareness, and rising domestic production are the main drivers of the immunoglobulin market in India. All these factors come together to increase demand and extend immunoglobulin therapy more widely across the country.
Growth Drivers for the India Immunoglobulin Market
Rising Prevalence of Immunodeficiency Disorders
One of the key drivers driving the growth of the Indian immunoglobulin market is the mounting cases of primary immunodeficiency diseases (PIDs). PIDs, a group of over 450 rare genetic conditions, weaken the immune system and expose individuals to increased susceptibility to infections, autoimmune disease, and cancer. In comparison to the worldwide incidence of 1 in 25,000 to 1 in 50,000 live births, India's estimated incidence of PIDs is 1 in 10,000 live births. Despite this higher prevalence, awareness and diagnosis remain poor, with as many as 70% of patients remaining undiagnosed, which increases morbidity and mortality rates. The sudden growth in unclassified cases highlights the need for awareness and early detection so critically. The demand for immunoglobulin therapy is driven by this growing patient base, which fuels market growth.
Advancements in Plasma-Derived Therapies
Plasma-derived treatments are a key driver of India's immunoglobulin market. The developments in plasma collection and processing technology have enhanced product quality and efficiency in production while reducing costs and making them more accessible. Immunoglobulin therapies are increasingly available due to these advances, along with increased domestic production and supportive government policies. This growth supports robust market growth and improved patient outcomes across the country by assisting in the alleviation of the increased demand generated by the increased incidence of autoimmune disorders and immunodeficiency in India.
Increasing Healthcare Awareness and Diagnosis Rates
India's immunoglobulin market is growing largely because there is increased healthcare awareness as well as improved diagnostic rates. As more individuals learn about autoimmune diseases and immunodeficiency disorders, increasingly more individuals are going to the doctor, thereby increasing the diagnoses. Immunoglobulin therapy is capable of being administered immediately due to this surge in early detection, which significantly improves patient outcomes. Adding to market demand even further is that advances in diagnostic technology have enhanced the accuracy and speed of detecting diseases treatable with immunoglobulins. More proactive approaches to health care and treatment access have been promoted by government schemes and medical campaigns, which have been essential to educate the public and medical professionals. Therefore, all these factors function jointly to facilitate the robust growth of the immunoglobulin market in India.
Challenges in the India Immunoglobulin Market
High Cost of Immunoglobulin Therapies
One major obstacle to the expansion of the immunoglobulin industry in India is the high expense of immunoglobulin treatments. These treatments produce pricey goods by requiring strict quality control and intricate manufacturing procedures. The high cost restricts accessibility and affordability for a large number of patients, particularly those from low-income and rural backgrounds. Furthermore, the reliance on imported plasma-derived goods drives up costs even more because of supply chain costs and import charges. This financial obstacle frequently results in incomplete or postponed therapy, which affects patient outcomes. Enhancing accessibility and propelling market expansion in India requires lowering therapeutic costs through better domestic production and encouraging government initiatives.
Regulatory and Quality Control Issues
Issues with quality control and regulations pose serious obstacles to the immunoglobulin business in India. Immunoglobulin products must adhere to strict regulatory criteria in order to be safe and effective, yet negotiating these convoluted clearance procedures can raise prices and delay market introduction. Furthermore, the diversity of plasma sources and production techniques makes it difficult to maintain uniform quality standards throughout manufacturing locations. Poor quality control can result in safety issues that undermine patient confidence and restrict market expansion. To overcome these obstacles and promote the sustainable growth of the Indian market, it is imperative to strengthen regulatory frameworks, improve inspection capacities, and encourage adherence to international quality standards.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 200 |
| Forecast Period | 2024-2033 |
| Estimated Market Value (USD) in 2024 | $347 Million |
| Forecasted Market Value (USD) by 2033 | $1.22 Billion |
| Compound Annual Growth Rate | 15% |
| Regions Covered | India |
Key Topics Covered
1. Introduction
2. Research & Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. India Immunoglobulin Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Market Share
6.1 By Product Type
6.2 By Mode of Delivery
6.3 By Application
6.4 By Region
7. Product Type
7.1 IgG
7.2 IgA
7.3 IgM
7.4 IgE
7.5 IgD
8. India Immunoglobulin Manufacturing Capacity
8.1 Biological E Limited
8.2 Intas Pharmaceuticals
8.3 Kedrion Biopharma
8.4 Bharat Serums and Vaccines
8.5 Reliance Life Sciences
9. Companies Investment Plans for Immunoglobulin Manufacturing in India
9.1 Plasma Collection Infrastructure/ Centres
9.2 Capital Requirements
10. Government Frameworks for Immunoglobulin Companies
10.1 Regulatory Framework
10.2 Pricing Control
11. Pricing Trends for IVIG Injections
11.1 Plasma Collection
11.2 Extraction and Purification
11.3 Formulation
11.4 Filling and Packaging
11.5 Regulations and Quality Control
12. Manufacturing Process for IVIG Injections
13. End-Users (Patient Numbers) of IVIG in India
13.1 Primary Immunodeficiency Diseases
13.2 Neurological Disorders
13.3 Hematological Disorders
13.4 Transplant Medicine
13.5 Autoimmune Diseases
14. Mode of Delivery
14.1 Intravenous Mode of Delivery
14.1.1 Historical Market Trends
14.1.2 Market Forecast
14.2 Subcutaneous Mode of Delivery
14.2.1 Historical Market Trends
14.2.2 Market Forecast
15. Application
15.1 Immunodeficiency Diseases
15.2 CIDP
15.3 Hypogammaglobulinemia
15.4 Congenital AIDS
15.5 Chronic Lymphocytic Leukemia
15.6 Myasthenia Gravis
15.7 Multifocal Motor Neuropathy
15.8 ITP
15.9 Kawasaki Disease
15.10 Others
16. Region
16.1 East
16.2 West
16.3 North
16.4 South
17. Porter's Five Analysis
17.1 Bargaining Power of Buyers
17.2 Bargaining Power of Suppliers
17.3 Degree of Rivalry
17.4 Threat of New Entrants
17.5 Threat of Substitutes
18. SWOT Analysis
18.1 Strength
18.2 Weakness
18.3 Opportunity
18.4 Threat
19. Company Analysis
19.1 Baxter international Inc.
19.2 Grifols SA
19.3 Bayer Healthcare
19.4 Takeda Pharmaceutical Company Limited
19.5 PlasmaGen BioSciences Pvt. Ltd.
19.6 Reliance Life Sciences
19.7 Biocon Limited
19.8 Virchow Biotech
19.9 Bharat Serums and Vaccines
19.10 Biological E Limited
19.11 Intas Pharmaceuticals
19.12 Kedrion Biopharma
For more information about this report visit https://www.researchandmarkets.com/r/860l9q
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
