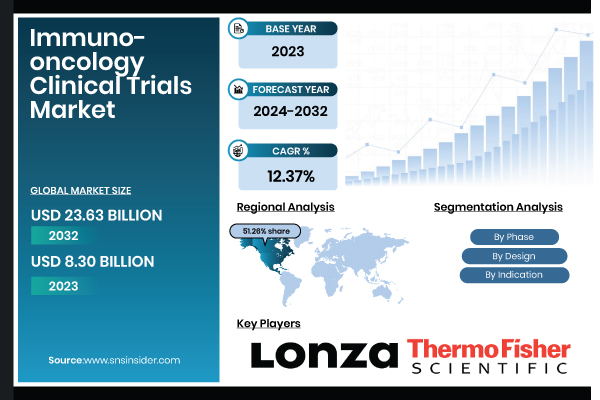
Austin, Texas, Dec. 14, 2025 (GLOBE NEWSWIRE) -- According to SNS Insider, The Immuno-oncology Clinical Trials Market size was valued at USD 8.30 billion in 2023 and is expected to reach USD 23.63 billion by 2032, growing at a CAGR of 12.37% from 2024-2032. The market is driven by the increasing global cancer burden, significant R&D investments, and the success of novel immunotherapies like checkpoint inhibitors and CAR-T cell therapies.
The U.S. Immuno-oncology Clinical Trials Market size was USD 3.08 billion in 2023 and is expected to reach USD 8.61 billion by 2032, growing at a CAGR of 12.15% over 2024-2032. The growing incidence of cancer and growing funding for immunotherapy research are driving the market's notable expansion. The need for clinical studies in this area has increased due to the emergence of checkpoint inhibitors, CAR-T cell treatments, and cancer vaccines.
Get a Sample Report of Immuno-oncology Clinical Trials Market: https://www.snsinsider.com/sample-request/6450
Rising Prevalence of Cancer is Driving the Market Expansion Globally
With pharmaceutical companies and research institutes placing a strong emphasis on innovative treatments, the growing worldwide cancer burden is a major factor propelling the immuno-oncology clinical trials market. According to the World Health Organization (WHO), lung, breast, and colorectal cancers were the most frequent types of cancer, accounting for about 10 million deaths in 2023. The need for novel immunotherapies such as checkpoint inhibitors, CAR-T cell therapy, and cancer vaccines has increased due to the disease's rising incidence.
High Cost and Complexity of Immuno-oncology Clinical Trials to Boost Market Growth Globally
The high expense and difficulty of conducting studies for novel cancer immunotherapies pose a barrier to the immuno-oncology clinical trials market. The development of checkpoint inhibitors, CAR-T cell treatments, and customized cancer vaccines requires extensive research, lengthy trial periods, and hefty operating costs, often exceeding USD 1 billion per medication. The problem is made more difficult by strict regulatory requirements and patient selection based on biomarkers.
Major Players Analysis Listed in the Immuno-oncology Clinical Trials Market Report are
- Merck and Co.
- Genmab
- Kite Pharma
- BeiGene
- Nanobiotix
- Imugene
- Novartis
- AstraZeneca
- BioNTech
- Gilead Sciences
- Bristol Myers Squibb
- Pfizer
- Roche
- Eli Lilly and Company
- Regeneron Pharmaceuticals
- Seagen
- Amgen
- Johnson and Johnson
- Celgene
- Takeda Pharmaceutical Company
Immuno-oncology Clinical Trials Market Report Scope
| Report Attributes | Details |
| Market Size in 2023 | US$ 8.30 Billion |
| Market Size by 2032 | US$ 23.63 Billion |
| CAGR | CAGR of 12.37 % From 2024 to 2032 |
| Base Year | 2023 |
| Forecast Period | 2024-2032 |
| Historical Period | 2020-2022 |
| Key Segments | • By Phase (Phase I, Phase II, Phase III, Phase IV) • By Design (Interventional Trials, Observational Trials, Expanded Access Trials) • By Indication (Solid Tumors, Hematological Cancer) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
Need Any Customization Research on Immuno-oncology Clinical Trials Market, Enquire Now: https://www.snsinsider.com/enquiry/6450
Segmentation Analysis:
By Phase
The Phase III segment dominated the Immuno-oncology Clinical Trials Market with 54.06% market share in 2023 due to the huge number of late-stage trials evaluating the safety and efficacy of immunotherapies before regulatory approval. The Phase I segment is expected to experience the fastest expansion during the forecast period, with the rise in first-stage investigation of new immunotherapies and second-generation immuno-oncology drugs.
By Design
The Interventional Trials segment dominated the Immuno-oncology Clinical Trials Market with a 79.40% market share during the year 2023 due to the rigorous testing nature of immuno-oncology drugs. The Observational Trials segment is experience to grow the fastest during the forecast period, owing to the increase in demand for real-world evidence (RWE) in immuno-oncology treatments.
By Indication
The Solid Tumors segment dominated the immuno-oncology clinical trials market with a 56.23% market share in 2023 due to the high prevalence of solid tumors, such as lung, breast, colorectal, and melanoma cancers globally.
Regional Insights:
North America dominated the immuno-oncology clinical trials market with a 51.26% market share in 2023, with its established clinical research landscape, strong regulatory regime, and extensive presence of major pharmaceutical and biotech firms.
With a 13.05% CAGR over the course of the projection year, Asia Pacific is witnessing the fastest growth in the immuno-oncology clinical trials market. This growth is being driven by increased engagement from pharmaceutical companies, rising cancer incidence, and growing spending in clinical research.
Statistical Insights and Market Trends
- The high global incidence of cancer continues to drive demand for immuno-oncology clinical trials, especially for checkpoint inhibitors, personalized vaccines, CAR T cell treatments, and TCR engineered therapies.
- Increased investment in biomarker validation and companion diagnostics is strengthening patient stratification and improving clinical outcomes across multiple cancer types.
- Regulatory agencies are expanding accelerated approval pathways, breakthrough therapy designations, and real-time review processes, which is improving approval efficiency and reducing time-to-market for promising therapies.
- Rising collaboration among biotechnology companies, academic research institutions, and government agencies is supporting intensive development of next-generation immunotherapies and combination regimens.
Purchase Single User PDF of Immuno-oncology Clinical Trials Market Report (20% Discount): https://www.snsinsider.com/checkout/6450
Recent Developments:
- February 2025: Merck KGaA, one of Germany's most prominent healthcare and technology companies, announced that it is in advanced talks to buy U.S.-based biopharmaceutical company SpringWorks Therapeutics. With expertise in cancer and rare disease treatments, the takeover of SpringWorks is expected to bolster Merck's pipeline of oncology medicines with a primary emphasis on advancing immuno-oncology treatments.
- September 2024: Clinical-stage biotech company Anbogen partnered with BeiGene to start a global Phase II trial. The trial will evaluate the effectiveness of a combination of Anbogen's HDAC inhibitor, ABT-301, with BeiGene's anti-PD-1 antibody, tislelizumab, in patients with mismatch repair–proficient or microsatellite stable metastatic colorectal cancer.
Exclusive Sections of the Report (The USPs):
- DRUG PIPELINE & SUCCESS RATE ANALYTICS – helps you evaluate the strength of the global immuno-oncology pipeline by tracking trial progression efficiency, approval probabilities, and pipeline maturity across major cancer therapeutics.
- GLOBAL TRIAL DISTRIBUTION INDEX – helps you understand regional clinical trial density, cross-region enrollment patterns, and emerging trial hotspots influencing investment and sponsor strategies.
- R&D INVESTMENT BENCHMARKS – helps you assess financial commitment across pharma, biotech, and research institutions by analyzing spending levels, funding trends, and long-term innovation priorities.
- BIOMARKER ADOPTION & PRECISION MEDICINE SCORE – helps you uncover the role of biomarker-driven patient selection, response prediction, and personalized therapy development in improving trial outcomes.
- REGULATORY APPROVAL & TIMELINE TRACKER – helps you evaluate how different regulatory bodies (FDA, EMA, PMDA, etc.) influence trial speed, approval probability, and market entry timelines.
- COMPETITIVE LANDSCAPE INSIGHTS – helps you gauge the strength of leading sponsors by analyzing trial volume, therapeutic focus, success rates, strategic collaborations, and advancement in next-generation immunotherapies.
Access Complete Report Details of Immuno-oncology Clinical Trials Market Analysis & Outlook: https://www.snsinsider.com/reports/immuno-oncology-clinical-trials-market-6450
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
