Austin, Texas, Dec. 16, 2025 (GLOBE NEWSWIRE) -- Viral Vectors and Plasmid DNA Manufacturing Market Size & Growth Analysis:
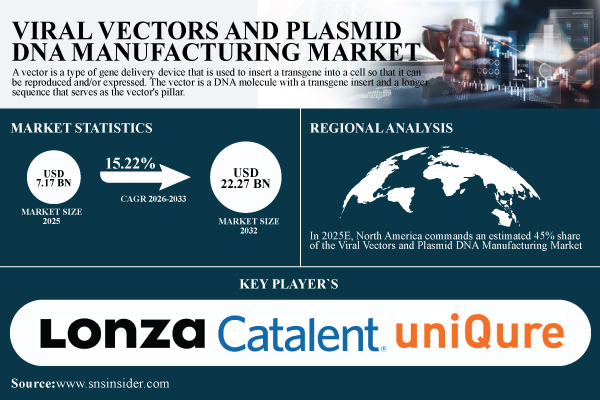
According to SNS Insider, the Viral Vectors and Plasmid DNA Manufacturing Market size is estimated at USD 7.17 billion in 2025 and is expected to reach USD 22.27 billion by 2032, growing at a CAGR of 15.22% over the forecast period of 2026–2033. The U.S. Viral Vectors and Plasmid DNA Manufacturing Market valued at USD 2.45 Billion in 2025 and is projected to reach USD 11.71 Billion by 2033.
Increasing adoption of gene and cell therapies, rising clinical trial activity, and the growing need for scalable, high-quality viral vector and plasmid DNA production are significantly contributing to market growth.
The Viral Vectors and Plasmid DNA Manufacturing Market is experiencing strong expansion due to the rapid advancement of gene therapy, cell therapy, and mRNA-based vaccines. Viral vectors such as adeno-associated virus (AAV), lentivirus, and adenovirus are widely used as delivery systems for genetic material, while plasmid DNA serves as a critical raw material for gene therapy vectors and mRNA vaccine production. These manufacturing processes require stringent GMP compliance, advanced upstream and downstream technologies, and high-quality analytical controls. As personalized medicine and rare disease therapies gain momentum, demand for reliable, large-scale manufacturing solutions continues to rise across biopharmaceutical companies and contract development and manufacturing organizations (CDMOs).
Get a Sample Report of Viral Vectors and Plasmid DNA Manufacturing Market: https://www.snsinsider.com/sample-request/3731
Market Overview:
The global viral vectors and plasmid DNA manufacturing market has witnessed robust growth due to increasing investments in gene and cell therapy research and commercialization. Viral vectors act as gene delivery vehicles that enable the insertion and expression of therapeutic genes in target cells, while plasmid DNA provides the foundational template for vector production and mRNA synthesis. The market is driven by the growing number of clinical trials, regulatory approvals for gene therapies, and the expansion of CDMO capabilities worldwide. Technological advancements such as single-use bioreactors, automated purification systems, and improved chromatography techniques have enhanced production efficiency, yield, and scalability. Additionally, strategic collaborations between pharmaceutical companies, biotechnology firms, and CMOs are accelerating capacity expansion and technology transfer. As demand for advanced therapeutics increases, the viral vectors and plasmid DNA manufacturing market is expected to witness sustained and rapid growth.
Major Players Analysis Listed in the Viral Vectors and Plasmid DNA Manufacturing Market Report are
- Thermo Fisher Scientific
- Lonza Group
- Catalent Inc.
- FUJIFILM Diosynth Biotechnologies
- Oxford Biomedica
- Aldevron (Danaher-owned)
- WuXi Advanced Therapies (WuXi AppTec)
- Charles River Laboratories
- VGXI Inc.
- Cobra Biologics
- SIRION Biotech GmbH
- PlasmidFactory GmbH
- AGC Biologics
- Yposkesi
- Takara Bio Inc.
- UniQure N.V.
- Virovek Incorporation
- RegenxBio, Inc.
- BioMarin Pharmaceutical
- MassBiologics
Viral Vectors and Plasmid DNA Manufacturing Market Report Scope
| Report Attributes | Details |
| Market Size in 2025E | US$ 7.17 Billion |
| Market Size by 2033 | US$ 22.27 Billion |
| CAGR | CAGR of 15.22 % From 2026 to 2033 |
| Base Year | 2025E |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Key Driver | Rising Demand for Gene and Cell Therapies Drives Growth in Viral Vectors and Plasmid DNA Manufacturing Market |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
Viral Vectors and Plasmid DNA Manufacturing Market Segmentation Analysis:
By Vector Type
The Adeno-Associated Virus (AAV) segment dominates with 29% of revenue in 2025E. AAV’s high safety profile, low immunogenicity, and long-term gene expression capabilities drive its widespread adoption in gene and cell therapies. The Lentivirus segment is growing at the fastest CAGR of 8.13% during the forecast period. Lentiviral vectors’ ability to transduce dividing and non-dividing cells makes them ideal for CAR-T and cell therapy pipelines.
By Workflow
The Downstream Purification segment dominates with 44% of revenue in 2025E. The growth is driven by the increasing demand for clinical- and commercial-grade products drives investment in chromatography, filtration, and automated downstream platforms. The Upstream Manufacturing segment is growing at the fastest CAGR of 9.89% during the forecast period. Rising demand for viral vectors and plasmid DNA for clinical and commercial applications drives investment in scalable cell culture, transfection, and production technologies.
By Application
The Vaccines segment dominates with 66% of revenue in 2025E. The urgent global need for viral-vector and mRNA-based vaccines drives large-scale plasmid DNA production, process optimization, and fill-finish operations. The Gene Therapy segment is growing at the fastest CAGR during the forecast period. Increasing approvals of AAV- and lentiviral-based therapies for rare genetic diseases and oncology expand demand for specialized viral vectors and GMP-grade plasmids.
By Grade
The GMP-Grade (Clinical) segment dominates with 42% of revenue in 2025E as clinical-grade plasmid DNA and viral vectors are critical for preclinical and early-phase trials, ensuring product quality and regulatory compliance. The GMP-Grade (Commercial) segment is growing at the fastest CAGR during the forecast period due to the increasing commercialization of gene therapies and viral-vector vaccines.
By End-User
The Academic & Research Institutes segment dominates with 42% of revenue in 2025E. Research institutions drive early-stage discovery, plasmid design, and viral vector development, generating significant demand for high-quality DNA and vectors. The Biopharmaceutical & Biotechnology Companies segment is growing at the fastest CAGR during the forecast period. Expanding gene and cell therapy portfolios, mRNA vaccines, and commercial vector therapies drive large-scale, GMP-compliant production needs.
Need Any Customization Research on Viral Vectors and Plasmid DNA Manufacturing Market, Enquire Now: https://www.snsinsider.com/enquiry/3731
Viral Vectors and Plasmid DNA Manufacturing Market Segmentation
By Vector Type
- Adenovirus
- Adeno-Associated Virus (AAV)
- Lentivirus
- Retrovirus
- Plasmids
- Others
By Workflow
- Upstream Manufacturing
- Downstream Purification
- Fill-Finish
- Quality Control & Testing
- Process Development
By Application
- Gene Therapy
- Cell Therapy (CAR-T, engineered cells)
- Vaccines
- Oncology / Oncolytic Viruses
- Research Use
By Grade
- Research-Grade
- GMP-Grade (Clinical)
- GMP-Grade (Commercial)
By End User
- Biopharmaceutical & Biotechnology Companies
- CDMOs / CMOs
- Academic & Research Institutes
- Vaccine Manufacturers
- Government / Public Health Agencies
Regional Insights:
Due to substantial expenditures in biopharmaceutical production, growing gene and cell therapy approvals, and sophisticated healthcare infrastructure, North America is expected to have a 45% share of the Viral Vectors and Plasmid DNA production Market in 2025E.
Asia Pacific is projected to grow at an estimated CAGR of 12.63%, fueled by expanding biotechnology investment, emerging gene therapy research, and increasing local CDMO capacities.
Recent Developments:
- In 2025, Thermo Fisher expanded its plasmid DNA production capacity in the U.S., introducing enhanced single-use bioreactors and automated purification platforms to support large-scale gene therapy and mRNA vaccine manufacturing.
- In June 2025, Lonza inaugurated a new viral vector manufacturing facility in Houston, Texas, designed to increase AAV and lentiviral vector output for clinical and commercial applications, leveraging advanced purification and automated analytics.
Purchase Single User PDF of Viral Vectors and Plasmid DNA Manufacturing Market Report (20% Discount): https://www.snsinsider.com/checkout/3731
Exclusive Sections of the Report (The USPs):
- CAPACITY UTILIZATION RATES – helps you identify whether viral vector and plasmid DNA manufacturing facilities are operating undercapacity or facing production bottlenecks, based on annual output volumes and utilization percentages.
- PROCESS EFFICIENCY & YIELD BENCHMARKS – helps you evaluate production performance through yield-per-liter metrics, batch success rates, and turnaround times, enabling comparison across CMOs and in-house facilities.
- REGULATORY COMPLIANCE METRICS – helps you understand GMP/FDA/EMA compliance levels, regulatory approval timelines, and annual QC failure counts across global manufacturing sites.
- COST & INVESTMENT ANALYTICS – helps you assess production costs per gram/dose, CAPEX spending trends on new biomanufacturing sites, and ROI expectations for technology or facility expansion.
- SUPPLY CHAIN DISRUPTION INDEX – helps you identify risk exposure related to critical raw materials (enzymes, media, cell lines) and evaluate regional vulnerabilities affecting vector and plasmid production.
- COMPETITIVE LANDSCAPE – helps you analyze the strategic positioning of leading manufacturers based on capacity scale, technology platforms, pipeline partnerships, and expansion announcements.
Access Complete Report Details of Viral Vectors and Plasmid DNA Manufacturing Market Analysis & Outlook: https://www.snsinsider.com/reports/viral-vectors-and-plasmid-dna-manufacturing-market-3731
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
