Dublin, Dec. 17, 2025 (GLOBE NEWSWIRE) -- The "United States Nucleic Acid Methylation Market Report by Product & Services, Type, Technology, Application, End Use and States and Company Analysis, 2025-2033" has been added to ResearchAndMarkets.com's offering.
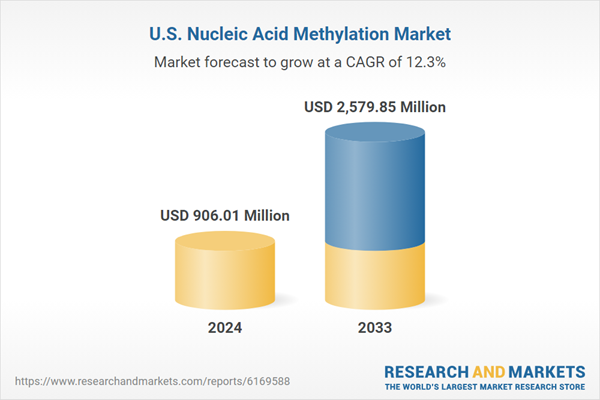
The US Nucleic Acid Methylation Market is estimated to expand substantially from US$ 906.01 million in 2024 to US$ 2.57 billion in 2033, registering a Compound Annual Growth Rate (CAGR) of 12.33% during the period 2025-2033. The expansion is fueled by more research in epigenetics, progress in methylation analysis technologies, and growing demand for personalized medicine, reflecting the increasing role of the market in the healthcare industry.
Efforts in precision medicine are fueling the growth of nucleic acid methylation technologies in the United States. Cancer continues to be a major cause of death, and DNA methylation profiles are key biomarkers for early diagnosis, prognosis, and monitoring of treatment response. U.S. research institutions, biotechnology firms, and hospitals are increasingly adopting methylation assays in oncology research to identify targeted therapies. Government support and academia-industry collaborations also augment this movement. The market demand for non-invasive liquid biopsy tests, which are methylation marker-based, is particularly vigorous, given that they provide less invasive, very precise diagnosis for patients.
May 2025: More than two million new cancer diagnoses and over 618,000 deaths are anticipated in the U.S. in 2025, the American Cancer Society predicts. To address the critical demand for focused therapies, the University of California San Diego School of Medicine has introduced a new graduate program: the Master of Advanced Studies in Precision Medicine Therapeutics in Oncology (PMTO). This interprofessional degree is prepared to arm healthcare providers, investigators, and life sciences professionals with the knowledge to drive innovation in precision medicine and enhance cancer treatment success.
The advent of next-generation sequencing (NGS) and advanced mass spectrometry equipment has brought methylation analysis to a higher level of accuracy, scalability, and affordability. US laboratories are employing high-throughput platforms for examining large genomic datasets, revealing epigenetic processes in cancer, autoimmune conditions, and neurological disorders. On-going advances in sequencing chemistry, bioinformatics, and automation improve the resolution of methylation profiling, facilitating broader adoption. They enable clinical researchers to translate findings into diagnostic tests and therapeutic approaches more rapidly, driving market growth.
Roche introduced in February 2025 a new sequencing by expansion (SBX) next-generation sequencing (NGS) technology. The technology applies expanded synthetic molecules to offer ultra-fast, scalable, and flexible DNA sequencing that is particularly crucial for complex diseases such as cancer.
Though oncology continues to be the biggest application, nucleic acid methylation is being researched in other therapeutic indications in the U.S. at growing length, such as cardiovascular disease, mental illness, metabolic disease, and age-related disease. Methylation biomarkers offer information about gene-environment interactions, allowing researchers to better understand disease risk and progression. The forensic sciences also utilize methylation assays for tissue origin identification and age estimation of biology. This widening of applications diversifies revenue potential and broadens the range of research support, making methylation technologies more appealing to healthcare providers, biotech companies, and diagnostic laboratories nationwide.
Although interest is increasing, nucleic acid methylation assays are still expensive, posing limitations for access by smaller labs and clinics in the United States. The cost of sequencing technologies, reagents, and computational tools may limit applications in diagnostics for routine use. Insurance coverage policies for epigenetic testing continue to mature, generating uncertainty for providers. These financial constraints delay extensive clinical implementation, especially for community hospitals or mini-diagnostics centers.
Methylation information is very complex and demands sophisticated bioinformatics to interpret. The absence of standardized lab protocols for sample preparation, data analysis, and reporting creates variability between laboratories. It becomes challenging to transfer study results into clinical practice in a consistent manner. In the United States, regulatory authorities and professional societies are only formulating guidelines, but the absence of common standards continues to be an enormous challenge to the scaling of clinical applications.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 200 |
| Forecast Period | 2024-2033 |
| Estimated Market Value (USD) in 2024 | $906.01 Million |
| Forecasted Market Value (USD) by 2033 | $2.57 Billion |
| Compound Annual Growth Rate | 12.3% |
| Regions Covered | United States |
Key Topics Covered
1. Introduction
2. Research & Methodology
2.1 Data Source
2.1.1 Primary Sources
2.1.2 Secondary Sources
2.2 Research Approach
2.2.1 Top-Down Approach
2.2.2 Bottom-Up Approach
2.3 Forecast Projection Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. United States Nucleic Acid Methylation Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Market Share Analysis
6.1 By Product & Services
6.2 By Type
6.3 By Technology
6.4 By Application
6.5 By End Use
6.6 By States
7. Product & Services
7.1 Kits & Reagents
7.2 Enzymes
7.3 Services
7.4 Instruments & Software
7.5 Consumables
8. Type
8.1 DNA Methylation
8.2 RNA Methylation
9. Technology
9.1 Next-Generation Sequencing (NGS)
9.2 Bisulflite Sequencing & PCR-based Techniques
9.3 Microarrat-based Methylation Analysis
9.4 Mass Spectrometry
9.5 Hybridization-based & Antibody-based Detection
10. Application
10.1 Drug Discovery & Personalized Medicines
10.2 Clinical Diagnostics
10.3 Others
11. End Use
11.1 Pharmaceutical & Biotechnology Companies
11.2 Academic & Research Institutes
11.3 Hospitals & Diagnostic Laboratories
12. Top States
12.1 California
12.2 Texas
12.3 New York
12.4 Florida
12.5 Illinois
12.6 Pennsylvania
12.7 Ohio
12.8 Georgia
12.9 New Jersey
12.10 Washington
12.11 North Carolina
12.12 Massachusetts
12.13 Virginia
12.14 Michigan
12.15 Maryland
12.16 Colorado
12.17 Tennessee
12.18 Indiana
12.19 Arizona
12.20 Minnesota
12.21 Wisconsin
12.22 Missouri
12.23 Connecticut
12.24 South Carolina
12.25 Oregon
12.26 Louisiana
12.27 Alabama
12.28 Kentucky
12.29 Rest of United States
13. Value Chain Analysis
14. Porter's Five Forces Analysis
14.1 Bargaining Power of Buyers
14.2 Bargaining Power of Suppliers
14.3 Degree of Competition
14.4 Threat of New Entrants
14.5 Threat of Substitutes
15. SWOT Analysis
15.1 Strength
15.2 Weakness
15.3 Opportunity
15.4 Threats
16. Pricing Benchmark Analysis
17. Key Players Analysis
17.1 New England Biolabs
17.2 Thermo Fisher Scientific Inc.
17.3 Illumina Inc.
17.4 Abcam plc
17.5 Agilent Technologies Inc.
17.6 F. Hoffmann-La Roche Ltd.
17.7 Bio-Rad Laboratories, Inc.
17.8 Exact Sciences Corporation
For more information about this report visit https://www.researchandmarkets.com/r/v17mij
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
