Dublin, Dec. 19, 2025 (GLOBE NEWSWIRE) -- The "Achondroplasia Treatment Market - Global Forecast 2025-2032" has been added to ResearchAndMarkets.com's offering.
The achondroplasia treatment market is evolving rapidly as new therapies, regulatory frameworks, and technological advances drive changes in both clinical care and organizational strategy. For senior decision-makers, understanding these shifts is crucial for optimizing investments and ensuring sustained competitive advantage in a complex global healthcare environment.
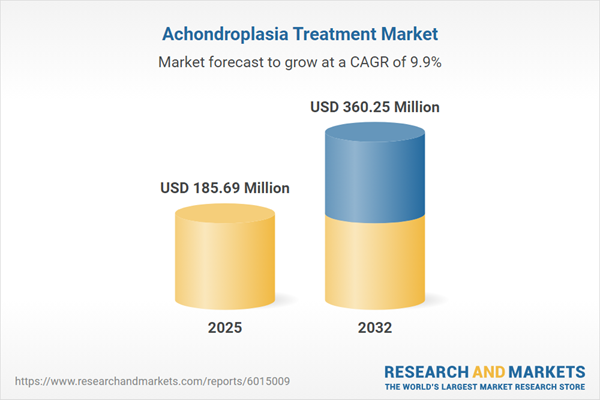
Market Snapshot: Achondroplasia Treatment Market Size and Growth
The Achondroplasia Treatment Market is experiencing robust expansion, with revenues projected to grow from USD 168.71 million in 2024 to USD 185.69 million in 2025, ultimately reaching USD 360.25 million by 2032. This growth translates to a compound annual growth rate (CAGR) of 9.94%. Key market drivers include the introduction of advanced biologic therapies, developments in surgical procedures, evolving reimbursement policies, and increased collaboration across the pharmaceutical and healthcare ecosystem. Emphasis on effective adoption of innovation is enhancing the sector's reach and strategic value worldwide, making this an opportune time for stakeholders to reevaluate their market positioning.
Scope & Segmentation: Executive Insights for the Achondroplasia Treatment Market
This report delivers targeted market intelligence for executives, offering a detailed view of opportunities and risks across the following core segments:
- Therapy Type: Covers biologic, small-molecule, and surgical approaches including limb lengthening and spinal decompression; also profiles emerging, patient-specific treatment options that support more targeted interventions.
- Patient Age Group: Investigates treatment modalities and management strategies for pediatric and adult populations, with special attention to protocols that optimize early intervention and guide long-term patient care planning.
- End User: Analyzes the impact that hospitals, specialty clinics, and research centers have on care delivery in multidisciplinary environments; outlines how streamlined pathways enhance both patient experiences and provider efficiency.
- Region Coverage: Compares healthcare system trends, technology adoption, and regulatory requirements in the Americas, Europe, Middle East & Africa, and Asia-Pacific; highlights regional challenges and diverse innovation patterns affecting market entry.
- Industry Participants: Features leading pharmaceutical organizations and cross-sector stakeholders, focusing on product development pipelines, strategic collaborations, and entry into both mature and high-growth healthcare markets.
Within these segments, the interplay between state-of-the-art technologies-such as precision surgical tools and integrated digital health solutions-impacts global care models and reimbursement structures. Understanding these factors is essential for executives aiming to balance timely therapy access, cost efficiencies, and superior patient outcomes across international markets.
Key Takeaways for Senior Decision-Makers
- Wider access to advanced biologic and peptide-based therapies is enabling more precise, patient-centered care, helping organizations adapt to diverse clinical requirements.
- Innovation in surgical methodology, particularly in limb lengthening and spinal care, is shaping practice standards and offering distinctive benefits for forward-thinking providers.
- Prioritizing early diagnosis and prompt therapeutic interventions, especially for pediatric patients, supports stronger long-term outcomes and guides strategic allocation of healthcare resources.
- Implementing multidisciplinary treatment models aligns with evolving value-based care frameworks, optimizing both provider workflows and the overall patient experience.
- Strategic partnerships and alliances are accelerating R&D timelines and expediting the commercialization of new therapeutic options, making organizational responsiveness increasingly valuable.
- Efforts to align regulatory practices are helping to streamline therapy approval processes, minimize cross-border complexity, and support more agile market entry.
Why This Report Matters: Strategic Decision Support
- Supports evidence-based decision-making by aligning executive strategies with dynamic clinical, regulatory, and market conditions across major healthcare regions.
- Provides integrated insights for mitigating risks, designing effective payer engagement plans, and optimizing resilient supply chains amid ongoing market changes.
- Delivers actionable recommendations for sustaining operational excellence and compliance in the context of evolving market and policy demands.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 190 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value (USD) in 2025 | $185.69 Million |
| Forecasted Market Value (USD) by 2032 | $360.25 Million |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
Companies Featured
The companies profiled in this Achondroplasia Treatment market report include:
- BioMarin Pharmaceutical Inc.
- Eli Lilly and Company
- Pfizer Inc.
- Novo Nordisk A/S
- Sandoz International GmbH
- Ipsen S.A.
- Ascendis Pharma A/S
For more information about this report visit https://www.researchandmarkets.com/r/44qu9o
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
