Dublin, Dec. 22, 2025 (GLOBE NEWSWIRE) -- The "Clinical Trial Supply & Logistics Market - Global Forecast 2025-2032" report has been added to ResearchAndMarkets.com's offering.
The clinical trial supply & logistics market is seeing transformative shifts driven by regulatory complexity, operational advances, and increased demand for patient-centric models. Senior leaders face evolving pressures that demand robust strategies to manage sourcing, distribution, compliance, and innovation.
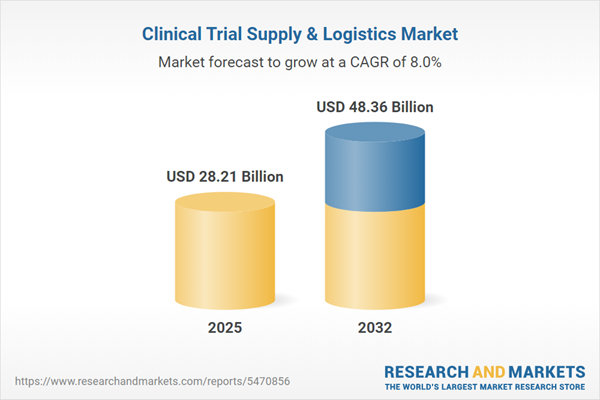
Market Snapshot: Clinical Trial Supply & Logistics Market Trends and Growth
The clinical trial supply & logistics market grew from USD 26.20 billion in 2024 to USD 28.21 billion in 2025. It is expected to continue growing at a CAGR of 7.96%, reaching USD 48.36 billion by 2032. Market momentum is being shaped by advances in digital tracking, increasingly decentralized trial models, and the rise of personalized medicine. In turn, sponsors are re-evaluating their relationships with logistics partners and pursuing integrated solutions that meet heightened global compliance expectations.
Scope & Segmentation
- Service Categories: Comparator sourcing, logistics and distribution (including cold chain and non-cold chain), manufacturing, packaging, labeling, blinding, storage, retention
- Sourcing Models: Centralized and decentralized sourcing approaches
- Product Types: Biologic drugs, medical devices, small molecules
- Mode of Delivery: Offsite and onsite supply management strategies
- End-User Profiles: Contract research organisations, medical device companies, pharmaceutical and biotechnology companies
- Therapeutic Areas: Oncology, infectious diseases, CNS disorders, rare diseases, dermatological, respiratory, cardiovascular, immunology, blood, digestive, nephrology, ENT, metabolic disorders
- Trial Phases: BA/BE studies, Phase 1, Phase 2, Phase 3, Phase 4
- Regions Covered: Americas (including North America and Latin America), Europe, Middle East & Africa, Asia-Pacific (spanning major and emerging markets)
- Companies Profiled: Almac Group Limited, Catalent, Inc., DHL Group, Eurofins Scientific SE, ICON PLC, Parexel International Corporation, SAP SE, Thermo Fisher Scientific Inc., and leading supply and logistics service providers, technology enablers, and regional distributors
Key Takeaways: Strategic Insights for Clinical Trial Supply Chain Leaders
- Technological adoption, including blockchain-enabled tracking and predictive analytics, is improving end-to-end supply visibility and risk mitigation potential.
- Regulatory demands for serialization, traceability, and labeling compliance are intensifying, requiring investment in automated solutions and centralized data management.
- Patient-centric and decentralized trial models are reshaping traditional supply chains, emphasizing direct-to-patient delivery and flexible cold chain distribution capabilities.
- Personalized treatment protocols are driving the need for small-batch manufacturing, customized packaging, and advanced blinding methods for diverse dosing regimens.
- Regional warehousing strategies and local partnerships support accelerated site activation and address regulatory fragmentation across global markets.
- Integrated service offerings that span comparator sourcing, packaging, logistics, and storage help streamline project management and deliver operational resilience.
Why This Report Matters
- Aligns market intelligence with emerging regulatory, technological, and operational demands facing senior decision-makers in clinical trial supply & logistics.
- Delivers actionable insights on best-practice sourcing, risk mitigation, and innovation strategies tailored to diverse global and regional requirements.
- Supports informed decision-making for investment, partnership, and competitive positioning in a rapidly evolving landscape.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 198 |
| Forecast Period | 2025-2032 |
| Estimated Market Value (USD) in 2025 | $28.21 Billion |
| Forecasted Market Value (USD) by 2032 | $48.36 Billion |
| Compound Annual Growth Rate | 7.9% |
| Regions Covered | Global |
Market Insights
- Implementation of IoT-enabled temperature monitoring systems for real-time cold chain compliance
- Adoption of decentralized clinical trial models driving demand for localized logistics networks
- Deployment of AI-driven predictive analytics for proactive inventory management in trials
- Increasing regulatory focus on serialisation and track-and-trace for investigational medicinal products
- Shift towards carbon neutral and sustainable packaging solutions in clinical trial logistics
- Use of advanced risk management platforms to ensure supply continuity amidst global disruptions
- Growth of on-demand direct-to-patient shipment models enhancing trial participant retention
- Integration of digital twin technologies for simulation and optimization of trial supply chains
- Rising leverage of cold chain robotics and automated storage systems for biologics transportation
- Focus on global harmonization of temperature excursion protocols to streamline multi-country trials
The companies profiled in this Clinical Trial Supply & Logistics market report include:
- Almac Group Limited
- Catalent, Inc.
- Acnos Pharma GmbH
- ADAllen Pharma Ltd
- Ancillare, LP
- Avantor, Inc.
- Beroe Holdings Inc.
- Biocair International Limited
- Calyx
- Eurofins Scientific SE
- Clinical Services International LTD
- Clinigen Group PLC
- COREX LOGISTICS LIMITED
- DHL Group
- Experic, LLC
- FedEx Corporation
- ICON PLC
- Inceptua S.A.
- Infosys Limited
- IPS Pharma
- KLIFO A/S
- Lonza Group AG
- Marken Limited by United Parcel Service, Inc.
- Microsoft Corporation
- Myonex, Inc.
- N-SIDE SA
- NUVISAN GmbH
- OCT Clinical GmbH
- Octalsoft
- Parexel International Corporation
- PCI Pharma Services
- PHOENIX Pharmahandel GmbH & Co KG
- Piramal Pharma Limited
- Recipharm AB
- SAP SE
- Sharp Services, LLC
- Signant Health
- SIRO Clinpharm Private Limited
- Thermo Fisher Scientific Inc.
- Tower Cold Chain Solutions
- Uniphar PLC
- Walden Group
- Zuellig Pharma Pte Ltd by Interpharma Investments Limited
For more information about this report visit https://www.researchandmarkets.com/r/2ps8bx
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
