Dublin, Dec. 29, 2025 (GLOBE NEWSWIRE) -- The "United States Medical Devices Cuffs Market Report by Type, End Use, States and Company Analysis, 2025-2033" has been added to ResearchAndMarkets.com's offering.
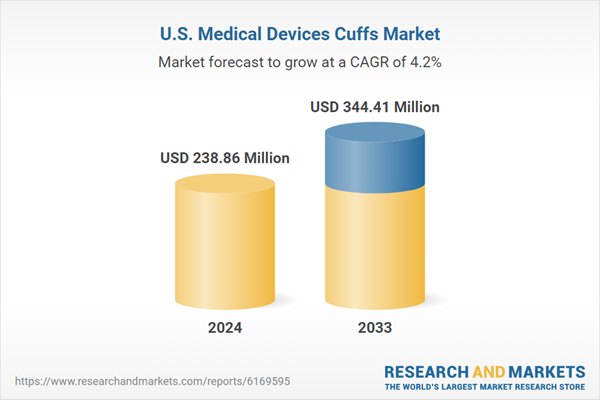
The United States Medical Devices Cuffs Market is expected to reach US$ 344.41 million by 2033 from US$ 238.86 million in 2024, with a CAGR of 4.15% from 2025 to 2033. The market for medical devices cuffs in the US is anticipated to grow gradually because of factors including growing healthcare demand, advancements in technology, and acceptance in clinics, hospitals, and homes for therapeutic and diagnostic uses.
The steady expansion of the medical device cuffs market in the United States is driven by the growing need for therapeutic and diagnostic instruments in healthcare institutions. Advanced cuff systems are being adopted by hospitals, clinics, and outpatient centers to guarantee precise diagnosis and effective patient care. The need for frequent monitoring solutions is growing as a result of the aging population, obesity, chronic diseases, and cardiovascular disorders. The market is growing because more people are adopting homecare as they look for practical, user-friendly medical gadgets for self-monitoring.
The rising incidence of hypertension and cardiovascular disorders, which call for routine blood pressure checks, is propelling the market's expansion. According to the WHO, 1.28 billion adults worldwide suffer from hypertension, a leading cause of premature death. Similarly, the CDC estimates that cardiovascular disease claims one American life every 33 seconds. In 2022, the nation saw 702,880 heart disease deaths, or almost one out of every five fatalities. In hospitals, clinics, and home healthcare settings, medical device cuffs - such as disposable and reusable blood pressure cuffs - are essential for precise diagnosis and patient monitoring. The market has grown as a result of an increase in the number of elderly people, who are more prone to chronic illnesses that call for constant blood pressure checks.
Furthermore, the prevalence of hypertension is rising significantly in a number of nations. In the United States, for example, the prevalence of hypertension rises with age. The prevalence was 23.4% among those aged 18-39, 52.5% among those aged 40-59, and 71.6% among those aged 60 and above. This age-related rise was seen in both males and females. Men were more likely than women to have hypertension in the younger (30.0% vs 16.4% for years 18-39) and middle-aged (55.9% vs 49.0% for ages 40-59) groups, but there was no discernible sex difference in the 60+ age group.
Key Factors Driving the United States Medical Devices Cuffs Market Growth
Rising Prevalence of Chronic Diseases and Aging Population
The growing prevalence of chronic diseases, particularly cardiovascular disorders, diabetes, and hypertension, is significantly driving the demand for medical devices cuffs in the United States. An aging population further contributes to this trend, as elderly individuals require regular monitoring and therapeutic interventions for age-related conditions. Blood pressure cuffs, compression cuffs, and therapeutic tourniquets are increasingly used in hospitals, clinics, and homecare settings to manage patient needs. Regular monitoring ensures timely diagnosis, prevention of complications, and improved patient outcomes. The aging demographic also creates demand for non-invasive, user-friendly devices that support self-care at home. With chronic diseases becoming more prevalent and the elderly population expanding, the adoption of medical devices cuffs is expected to remain a cornerstone of patient monitoring and treatment strategies across the nation.
Technological Advancements and Integration with Digital Health
Technological innovation is a major driver of growth in the U.S. medical devices cuffs market. Modern devices are moving beyond traditional manual designs, incorporating automation, wireless connectivity, and smart sensors to improve accuracy and usability. Integration with digital health platforms allows real-time monitoring, enabling physicians and caregivers to track patient data remotely and provide timely interventions. Wearable cuff technologies and AI-driven analytics are also enhancing diagnostic precision while promoting patient engagement. These innovations support the shift toward preventive care, reduce hospital readmissions, and improve healthcare efficiency. By aligning with telemedicine services and remote patient management trends, advanced medical cuffs are transforming how healthcare providers monitor patients. As technology continues to evolve, it is driving widespread adoption and shaping the future of cuff-based medical solutions.
Expanding Homecare and Outpatient Monitoring Adoption
The shift toward homecare and outpatient monitoring is a key factor accelerating the growth of the U.S. medical devices cuffs market. Patients increasingly prefer monitoring their health from home, reducing the need for frequent hospital visits. Blood pressure cuffs, compression therapy devices, and rehabilitation cuffs are widely adopted in homecare environments due to their ease of use and reliability. Outpatient facilities also utilize cuffs for regular monitoring of chronic disease patients, supporting efficient care delivery. The rising popularity of telehealth services has reinforced this trend, as devices can seamlessly transmit data to healthcare providers for evaluation. This convenience improves patient compliance, reduces healthcare costs, and enhances overall outcomes. The growing demand for home-based healthcare solutions is positioning medical devices cuffs as essential tools in modern healthcare management.
Challenges in the United States Medical Devices Cuffs Market
High Costs and Affordability Issues
High costs of advanced medical devices cuffs pose a significant challenge in the U.S. market. While modern devices with wireless, automated, and smart features offer improved accuracy and convenience, they often come with premium price tags. Smaller clinics, rural healthcare facilities, and some homecare users may find it difficult to invest in these devices. In addition, ongoing maintenance, calibration, and replacement costs add to the financial burden, limiting widespread adoption. The affordability gap can create disparities in access to advanced diagnostic tools, particularly in underserved regions. Although low-cost alternatives exist, they may lack the accuracy and durability of advanced models. Addressing affordability through cost-effective solutions, financing options, and supportive reimbursement policies will be crucial to expanding market reach and ensuring equitable healthcare access.
Regulatory Compliance and Standardization Challenges
Regulatory compliance and product standardization present ongoing challenges for the medical device's cuffs market in the United States. Devices used for diagnostic and therapeutic purposes must meet stringent safety and performance standards enforced by agencies such as the FDA. Ensuring calibration accuracy, consistent performance, and safety features requires manufacturers to invest heavily in testing and certification, increasing development costs and timelines. Variability in device quality across manufacturers also raises concerns regarding standardization, particularly in homecare settings where professional oversight is limited. Healthcare providers and patients may face difficulties in selecting devices that meet both safety and performance requirements. Balancing innovation with compliance is essential, as regulatory hurdles can delay product launches and adoption. Addressing standardization challenges will be vital to ensuring reliable and safe patient care.
Key Attributes
| Report Attribute | Details |
| No. of Pages | 200 |
| Forecast Period | 2024-2033 |
| Estimated Market Value (USD) in 2024 | $238.86 Million |
| Forecasted Market Value (USD) by 2033 | $344.41 Million |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | United States |
Key Topics Covered
1. Introduction
2. Research & Methodology
3. Executive Summary
4. Market Dynamics
4.1 Growth Drivers
4.2 Challenges
5. United States Medical Devices Cuffs Market
5.1 Historical Market Trends
5.2 Market Forecast
6. Market Share Analysis
6.1 By Type
6.2 By End Use
6.3 By States
7. Type
7.1 Blood Pressure Cuffs
7.2 Cuffed Endotracheal Tube
7.3 Tracheostomy Tube
8. End Use
8.1 Hospitals
8.2 Clinics
8.3 Ambulatory Surgery Centers
8.4 Others
9. Top States
9.1 California
9.2 Texas
9.3 New York
9.4 Florida
9.5 Illinois
9.6 Pennsylvania
9.7 Ohio
9.8 Georgia
9.9 New Jersey
9.10 Washington
9.11 North Carolina
9.12 Massachusetts
9.13 Virginia
9.14 Michigan
9.15 Maryland
9.16 Colorado
9.17 Tennessee
9.18 Indiana
9.19 Arizona
9.20 Minnesota
9.21 Wisconsin
9.22 Missouri
9.23 Connecticut
9.24 South Carolina
9.25 Oregon
9.26 Louisiana
9.27 Alabama
9.28 Kentucky
9.29 Rest of United States
10. Value Chain Analysis
11. Porter's Five Forces Analysis
11.1 Bargaining Power of Buyers
11.2 Bargaining Power of Suppliers
11.3 Degree of Competition
11.4 Threat of New Entrants
11.5 Threat of Substitutes
12. SWOT Analysis
12.1 Strength
12.2 Weakness
12.3 Opportunity
12.4 Threats
13. Pricing Benchmark Analysis
14. Key Players Analysis
14.1 Cardinal Health
14.2 ConvaTec Inc.
14.3 Cook Medical
14.4 GE Healthcare
14.5 Omron Healthcare Inc.
14.6 Pulmodyne Inc.
14.7 Smiths Medical
14.8 SunTech Medical Inc.
14.9 Teleflex Inc.
14.10 Welch Allyn Inc.
For more information about this report visit https://www.researchandmarkets.com/r/m3hizx
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
