Dublin, Jan. 06, 2026 (GLOBE NEWSWIRE) -- The "Artificial Intelligence (AI) in Regulatory Affairs Global Market Report 2025" has been added to ResearchAndMarkets.com's offering.
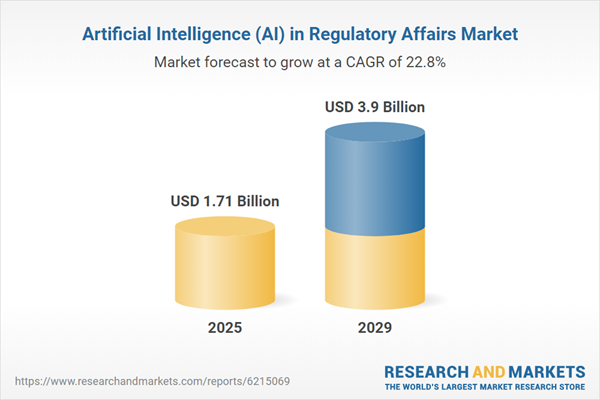
The artificial intelligence (AI) in regulatory affairs market is poised for significant growth, with its size expected to expand from $1.39 billion in 2024 to $1.71 billion by 2025, reflecting a compound annual growth rate (CAGR) of 23.2%. This momentum is driven by increasing global regulatory complexities, widespread adoption of AI technologies in life sciences, and a pressing need for expedited drug approval processes.
Forecasts indicate that the market value will reach $3.9 billion in 2029, maintaining a CAGR of 22.8%. Factors contributing to this growth include a focus on minimizing human error in regulatory submissions, strengthened collaboration between regulatory bodies and tech firms, and a rising emphasis on data-driven regulatory decision-making.
AI-enabled platforms are integral to this growth, as they automate complex regulatory tasks, accelerate data processing, and ensure precise decision-making. These solutions support faster regulatory approvals and enhance data accuracy, thus driving innovation and market readiness. Notably, the UK's AI sector generated USD 16.32 billion in 2023, marking a 34% year-on-year increase, highlighting a substantial rise in employment within AI-related roles.
Leading companies are investing in advanced innovations such as Regulatory Information Management (RIM) systems. A prime example is the July 2024 launch of ArisGlobal LLC's upgraded LifeSphere Regulatory Platform, a comprehensive cloud-based system that integrates next-generation GenAI capabilities for enhanced regulatory affairs management.
The sector has also witnessed strategic acquisitions aimed at enhancing capabilities, as evidenced by Ernst & Young Global Limited's acquisition of Aqurance A.E. in October 2025. This move bolsters EY's offerings in life sciences consulting, enabling improved AI-driven regulatory solutions.
Prominent players dominating the AI in regulatory affairs market include Freyr Software Services, Celegence LLC, Wipro Limited, Indegene Limited, and IBM among others. As North America leads the market, Asia Pacific is projected to experience the fastest growth through the forecast period.
However, the industry faces challenges due to global trade dynamics. The escalation of U.S. tariffs in 2025 significantly impacts healthcare, disrupting the supply chain of medical devices and pharmaceuticals, and inflating costs for healthcare providers. The industry response has been to adapt sourcing strategies and boost local manufacturing to mitigate these effects.
The market research report on AI in regulatory affairs offers in-depth analysis, covering regional shares, market trends, and potential opportunities to equip stakeholders with comprehensive insights for strategic decision-making in this rapidly evolving field.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 250 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value (USD) in 2025 | $1.71 Billion |
| Forecasted Market Value (USD) by 2029 | $3.9 Billion |
| Compound Annual Growth Rate | 22.8% |
| Regions Covered | Global |
Report Scope:
Markets Covered:
- By Component: Software or Platforms; Services
- By Deployment Mode: Cloud-based; On-Premises
- By Application: Regulatory Intelligence; Data Migration and Integration; Dossier Management; Product Registration and Approvals; Pharmacovigilance and Safety Reporting; Regulatory Submissions and Publishing; Other Applications
- By End-User: Pharmaceutical Companies; Biotechnology Companies; Other End-Users
Subsegments:
- By Software or Platforms: Regulatory Information Management; Document Management; Data Analytics and Insights; Natural Language Processing Tools; Cloud-Based Compliance Solutions; Workflow Automation Systems; AI-Powered Decision Support
- By Services: Implementation and Integration; Training and Consulting; Support and Maintenance; Regulatory Process Outsourcing; Data Migration and Validation; Compliance Monitoring Services; Managed Services
Companies Featured
- Freyr Software Services Private Limited
- Celegence LLC
- Wipro Limited
- Indegene Limited
- Clarivate Plc
- Zenovel Pharma Services LLP
- RegDesk Inc
- Compliance.ai Inc
- IONI AI INC.
- Interfacing Technologies Corporation
- OneTrust LLC
- Xapien Ltd.
- SpringsApps Technologies Pvt Ltd.
- Fairnow Inc.
- Navitas Life Sciences Private Limited
- S&P Global Inc.
- International Business Machines Corporation
- ZS Associates International Inc.
- Vistaar Technologies Inc.
- Lexim AI Inc.
For more information about this report visit https://www.researchandmarkets.com/r/8rix4r
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
