
SOUTH SAN FRANCISCO, Calif., Feb. 11, 2026 (GLOBE NEWSWIRE) -- Deep Origin, the computational drug discovery company replacing guesswork with prediction in small molecule drug development, today congratulated Isomorphic Labs on the unveiling of their Drug Design Engine (IsoDDE) and its reported advances on the Runs N’ Poses protein-ligand docking benchmark.
We are glad that Isomorphic is helping advance the field and are able to match the level of performance we demonstrated at the ARDD conference in Copenhagen last August.
In August, Deep Origin Co-Founder and CEO Michael Antonov presented results at the Aging Research and Drug Discovery (ARDD) 2025 conference showing that DODock, the company’s physics-informed docking engine, greatly outperformed AlphaFold 3 and other leading docking methods across low-similarity systems on the same Runs N’ Poses benchmark, where prior structure prediction models failed to generalize.
Last fall, at the AI/ML for Early Drug Discovery Chemistry conference in Barcelona, the Deep Origin team presented expanded results including prospective drug discovery data demonstrating the real-world impact of these tools.
“We’re glad to see Isomorphic Labs reaching performance levels comparable to what we presented months ago. More accurate docking is good for drug discovery, full stop,” said Antonov. “But benchmarks are table stakes. The real question is how these tools are used to actually find drugs, and whether you can get your docking in the hands of many scientists—and we’re doing that.”
Benchmark Success
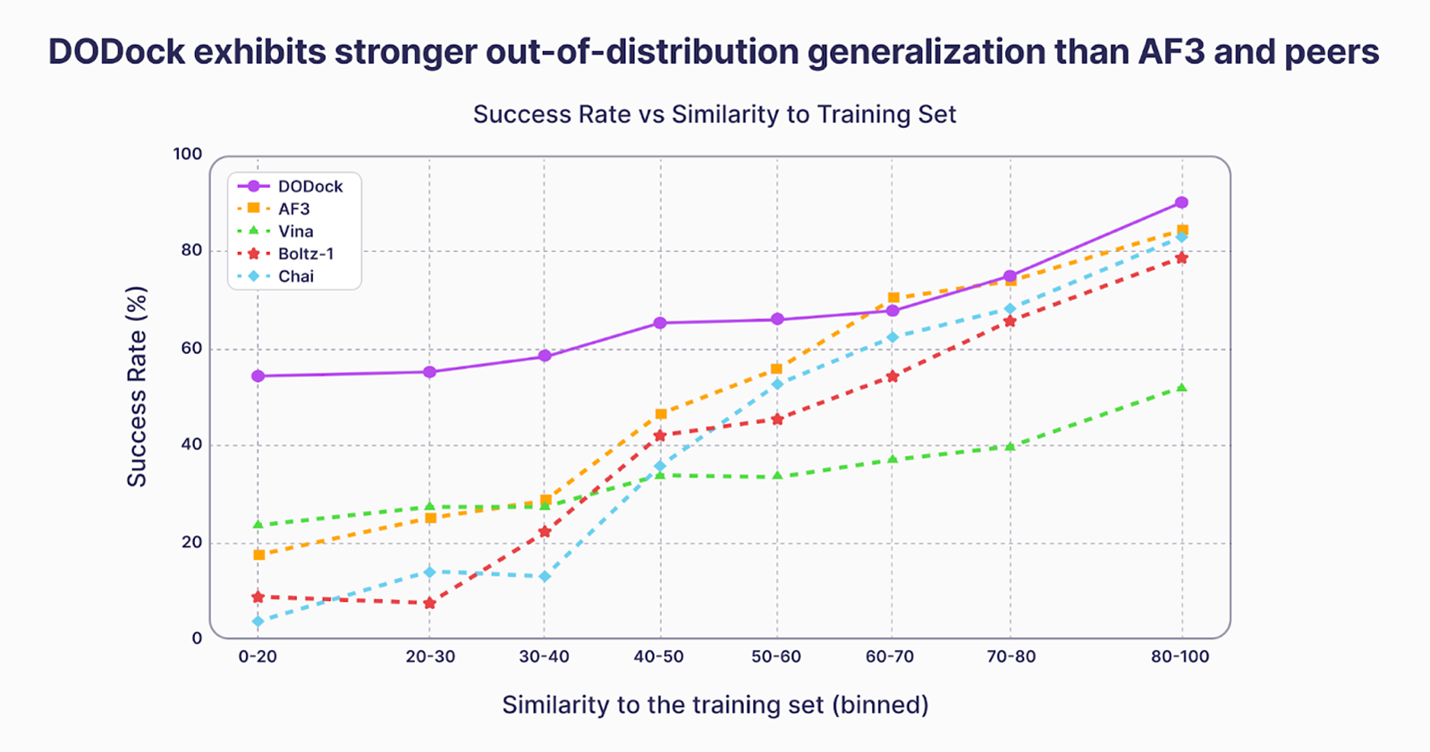
Isomorphic Labs reports that IsoDDE “more than doubles” AlphaFold 3’s accuracy systems less similar to the training set data. Deep Origin’s DODock achieved comparable performance on this same benchmark last year, using a fundamentally different approach: combining atomistic physics-based models with machine learning rather than relying on AI co-folding alone. On the separate PoseBusters benchmark, DODock exceeded a 90% success rate.
“Our approach with DODock combines atomistic physics with machine learning because we're practical: we use what works best, based on what we are trying to achieve,” said Dr. Garegin Papoian, Deep Origin’s Co-Founder and Chief Scientific Officer. “What matters is that these tools move us closer to a true engineering discipline in drug discovery, where you design a molecule computationally and it works the way you designed it. Benchmarks are one proof point. Our prospective drug discovery results are another.”
From Benchmarks to Real Drug Discovery
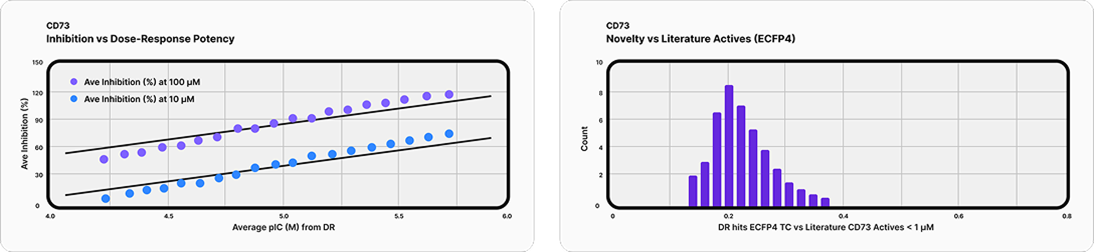
Deep Origin has already applied its docking engine to prospective drug discovery on difficult real-world targets—with striking results. At the Drug Discovery Chemistry conference in Barcelona, Deep Origin’s team reported that it had screened approximately 9 billion compounds from the Enamine REAL database plus roughly 71 billion from unenumerated REAL Space to identify candidates against CD73 (5’-nucleotidase NT5E)—a high-value immuno-oncology target where prior virtual screening campaigns have been largely unsuccessful ¹ ². Of 160 diverse candidates selected for experimental testing, 48 showed activity below 80 µM and 9 demonstrated potency below 10 µM, with broad chemotype diversity across the hit set.
Access, Not Just Accuracy
Yesterday’s announcement from Isomorphic Labs was a technical report disclosing results from an internal tool that remains available only within Isomorphic’s partnerships. Deep Origin is building toward a different model.
DODock will launch publicly later in H1 (if you’re interested in early access, we’re accepting users on a case-by-case basis here), joining the company’s growing suite of tools including Balto AI and DO Patent. Deep Origin’s platform is accessible through both a code-based Python API and a chat-based natural-language interface designed to put predictive computational chemistry directly in the hands of medicinal chemists—at organizations of any size.
“So to our friends at Isomorphic Labs: nice work catching up on the docking benchmark,” said Antonov. “Now it’s up to all of us to get these technologies to the people doing drug discovery work.”
About Deep Origin
Deep Origin is a computational drug discovery company working to replace uncertainty with computational precision in small molecule drug discovery and development. Co-founded by Michael Antonov, co-founder of Oculus, and Dr. Garegin Papoian, a computational chemist whose BioSim.ai team brought decades of expertise in physics-based molecular simulation, the company builds AI and physics-based tools that optimize key steps from hit finding through preclinical development. Learn more at deeporigin.com.
Citations
1. The Atomwise AIMS Program. AI is a viable alternative to high throughput screening: a 318-target study. Sci Rep 14, 7526 (2024). https://doi.org/10.1038/s41598-024-54655-z
2. Kumar M, Lowery R, Kumar V. High-Throughput Screening Assays for Cancer Immunotherapy Targets: Ectonucleotidases CD39 and CD73. SLAS Discov. 2020 Mar;25(3):320-326. https://doi.org/10.1177/2472555219893632
Contact: Dan Boyle
dan@orangefiery.com | (818) 209-1692
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/4d71b787-deb7-43a2-8876-3864ec4c2f5d
https://www.globenewswire.com/NewsRoom/AttachmentNg/648fa896-08df-43aa-b405-d65c9a1d3281
https://www.globenewswire.com/NewsRoom/AttachmentNg/5f407a21-f482-4c21-8ce0-ed16831975b5
