-The first 4 Patients enrolled into Cohort 1 of the BB-301 Phase 1b/2a treatment study have completed the 12-month statistical follow-up period, and all 4 Completers were formal Responders to BB-301 at the 12-month follow-up timepoint demonstrating durable response to BB-301-
-Patient 1 of Cohort 1 completed the 24-month follow-up timepoint, and at the 24-month post-treatment timepoint Patient 1 continued to experience the disease-modifying effects of BB-301, with deepening improvements in post-swallow residue and total dysphagic symptom burden as compared to the 12-month follow-up timepoint-
- An update on the Interim clinical results for Cohort 2 is planned for mid-2026-
-FDA meeting to formalize the pivotal BB-301 study design expected mid-year-
HAYWARD, Calif.,, Feb. 12, 2026 (GLOBE NEWSWIRE) -- Benitec Biopharma Inc. (NASDAQ: BNTC) (“Benitec” or the “Company”), a clinical-stage, gene therapy-focused, biotechnology company developing novel genetic medicines based on its proprietary “Silence and Replace” DNA-directed RNA interference ("ddRNAi") platform, today announced financial results for its second fiscal quarter ended December 31, 2025. The Company has filed its quarterly report on Form 10-Q with the U.S. Securities and Exchange Commission.
“We continue to be encouraged by the benign safety profile and the durability of efficacy demonstrated in our BB-301 clinical development program,” said Jerel A. Banks, M.D., Ph.D., Executive Chairman and Chief Executive Officer of Benitec. “We look forward to engaging with the U.S. Food and Drug Administration (FDA) in mid-2026 to confirm the BB-301 pivotal study design and continuing to present interim clinical results at future medical conferences. I want to sincerely thank our investigators, our clinical advisors, and—most importantly—the patients and families who have made this progress possible.”
The recent and upcoming key milestones related to the development of BB-301 for the treatment of Oculopharyngeal Muscular Dystrophy-related Dysphagia, are outlined below:
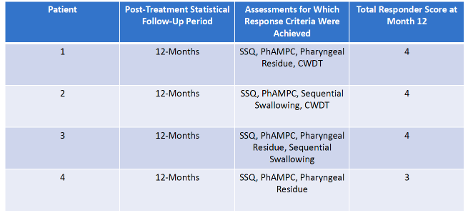
Responder Analysis for Study Completers
- A Responder Analysis was developed to facilitate standardized evaluation of BB-301 efficacy for each Patient. The Responder Analysis consists of multiple discrete response categories that collectively assess the dysphagic symptom burden in patients with OPMD.
- These response categories include:
- Patient-Reported Outcome: Patient-reported oral-pharyngeal dysphagia as assessed by the Sydney Swallow Questionnaire (SSQ) total score
- Videofluoroscopic Swallowing Study (VFSS) Assessments:
- Pharyngeal constrictor muscle function as estimated by the Pharyngeal Area at Maximum Constriction (PhAMPC)
- Swallowing efficiency as measured by NRRSv and Total Pharyngeal Residue %(C2-4)2
- Frequency of pathologic sequential swallows (SEQ)
- Functional Swallowing Capacity: Cold-Water Timed Drinking Test (CWDT)
- The evaluation is completed as follows:
- Following completion of the 12-month post-treatment follow-up timepoint, each discrete response category is evaluated for each study Completer using prespecified statistical criteria
- Results of the statistical characterization of each response category are combined into a single scoring framework that facilitates the overall assessment of clinical benefit achieved by each Completer following treatment with BB-301
- A total Score of 5 is possible
- Responder status for each Completer will be assigned based on the achievement of statistical criteria for at least 2 out of 5 discrete response categories (≥40%)
Responder Analysis for Study Completers:
All four Cohort 1 Completers were formal responders to BB-301, demonstrating durable response to BB-301 at the conclusion of the 12-month statistical follow-up period.
24-Month Post-Treatment Follow-Up for Patient 1 of Cohort 1
At the 24-month post-BB-301 treatment follow-up timepoint, Patient 1 of Cohort 1 continued to demonstrate robust, disease-modifying outcomes. Patient 1 demonstrated deepening improvements in post-swallow pharyngeal residue as compared to the final pre-treatment timepoint and as compared to the 12-month post-treatment follow-up timepoint as assessed by VFSS. Patient 1 also experienced deepening improvements in total dysphagic symptom burden as assessed by the SSQ.
Enrollment into the BB-301 Phase 1b/2a Clinical Treatment Study is Ongoing:
The first Patient in Cohort 2 was safely treated with the higher-dose of BB-301 in 4Q of 2025 and an update on the interim clinical results of Cohort 2 is planned for mid-2026.
Corporate Updates:
The Company plans to engage with the FDA in mid-2026 to confirm the BB-301 pivotal study design. In November 2025, Fast Track Designation was granted for BB-301 following FDA review of positive interim clinical study results and the proprietary Responder Analysis planned for use in the BB-301 pivotal study. Previously, BB-301 has received Orphan Drug Designation from the EMA and the FDA.
Financial Highlights
Second Quarter 2026 Financial Results
Total Expenses for the quarter ended December 31, 2025 were $13.4 million compared to $10.8 million for the quarter ended December 31, 2024. The Company incurred $5.8 million of research and development expenses which was in line with $5.4 million for the comparable quarter ended December 31, 2024. Research and development expenses relate primarily to ongoing clinical development of BB-301 for the treatment of OPMD. General and administrative expenses were $7.5 million compared to $5.4 million for the quarter ended December 31, 2024.
The loss from operations for the quarter ended December 31, 2025, was $13.4 million compared to a loss of $10.8 million for the quarter ended December 31, 2024. Net loss attributable to shareholders for the quarter ended December 31, 2025, was $11.8 million, or $(0.26) per basic and diluted share, compared to a net loss of $9.6 million, or $(0.26) per basic and diluted share for the quarter ended December 31, 2024. As of December 31, 2025, the Company had $189 million in cash and cash equivalents, which includes $0.1 million from the exercise of warrants during the six-month period ended December 31, 2025.
| BENITEC BIOPHARMA INC. Consolidated Balance Sheets (in thousands, except par value and share amounts) | ||||||||
| December 31, 2025 | June 30, 2025 | |||||||
| (Unaudited) | ||||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 188,790 | $ | 97,744 | ||||
| Restricted cash | 113 | 113 | ||||||
| Trade and other receivables | 130 | 33 | ||||||
| Prepaid and other assets | 562 | 628 | ||||||
| Total current assets | 189,595 | 98,518 | ||||||
| Property and equipment, net | 115 | 131 | ||||||
| Deposits | 55 | 55 | ||||||
| Prepaid and other assets | 11 | 28 | ||||||
| Right-of-use assets | 905 | 860 | ||||||
| Total assets | $ | 190,681 | $ | 99,592 | ||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Trade and other payables | $ | 1,850 | $ | 1,022 | ||||
| Accrued employee benefits | 483 | 426 | ||||||
| Lease liabilities, current portion | 468 | 354 | ||||||
| Total current liabilities | 2,801 | 1,802 | ||||||
| Lease liabilities, less current portion | 519 | 495 | ||||||
| Total liabilities | 3,320 | 2,297 | ||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $0.0001 par value—5,000,000 shares authorized; no shares issued and outstanding at December 31, 2025 and June 30, 2025, respectively | — | — | ||||||
| Common stock, $0.0001 par value—160,000,000 shares authorized; 34,254,907 and 26,250,469 shares issued and outstanding at December 31, 2025 and June 30, 2025, respectively | 3 | 2 | ||||||
| Additional paid-in capital | 437,219 | 326,308 | ||||||
| Accumulated deficit | (248,978 | ) | (228,176 | ) | ||||
| Accumulated other comprehensive loss | (883 | ) | (839 | ) | ||||
| Total stockholders’ equity | 187,361 | 97,295 | ||||||
| Total liabilities and stockholders’ equity | $ | 190,681 | $ | 99,592 | ||||
| BENITEC BIOPHARMA INC. Consolidated Statements of Operations and Comprehensive Loss (Unaudited) (in thousands, except share and per share amounts) | ||||||||||||||||
| Three Months Ended | Six Months Ended | |||||||||||||||
| December 31, | December 31, | |||||||||||||||
| 2025 | 2024 | 2025 | 2024 | |||||||||||||
| Revenue: | ||||||||||||||||
| $ | — | $ | — | $ | — | $ | — | |||||||||
| Total revenues | — | — | — | — | ||||||||||||
| Operating expenses | ||||||||||||||||
| Research and development | 5,834 | 5,385 | 9,204 | 8,970 | ||||||||||||
| General and administrative | 7,543 | 5,420 | 13,976 | 7,626 | ||||||||||||
| Total operating expenses | 13,377 | 10,805 | 23,180 | 16,596 | ||||||||||||
| Loss from operations | (13,377 | ) | (10,805 | ) | (23,180 | ) | (16,596 | ) | ||||||||
| Other income (loss): | ||||||||||||||||
| Foreign currency transaction gain (loss) | 131 | (294 | ) | 42 | (201 | ) | ||||||||||
| Interest income, net | 1,390 | 823 | 2,401 | 1,427 | ||||||||||||
| Other income (expense), net | 19 | (40 | ) | (65 | ) | (5 | ) | |||||||||
| Gain on extinguishment of liabilities | — | 764 | — | 764 | ||||||||||||
| Total other income, net | 1,540 | 1,253 | 2,378 | 1,985 | ||||||||||||
| Net loss | $ | (11,837 | ) | $ | (9,552 | ) | $ | (20,802 | ) | $ | (14,611 | ) | ||||
| Other comprehensive income: | ||||||||||||||||
| Unrealized foreign currency translation gain (loss) | (133 | ) | 305 | (44 | ) | 204 | ||||||||||
| Total other comprehensive income (loss) | (133 | ) | 305 | (44 | ) | 204 | ||||||||||
| Total comprehensive loss | $ | (11,970 | ) | $ | (9,247 | ) | $ | (20,846 | ) | $ | (14,407 | ) | ||||
| Net loss | $ | (11,837 | ) | $ | (9,552 | ) | $ | (20,802 | ) | $ | (14,611 | ) | ||||
| Net loss attributable to common shareholders | $ | (11,837 | ) | $ | (9,552 | ) | $ | (20,802 | ) | $ | (14,611 | ) | ||||
| Net loss per share: | ||||||||||||||||
| Basic and diluted | $ | (0.26 | ) | $ | (0.26 | ) | $ | (0.48 | ) | $ | (0.45 | ) | ||||
| Weighted average number of shares outstanding: basic and diluted | 45,970,516 | 37,254,839 | 43,745,898 | 32,574,158 | ||||||||||||
About BB-301
BB-301 is a silence and replace-based genetic medicine currently under development by Benitec. BB-301 uses DNA-directed RNA interference (ddRNAi) to simultaneously silence the mutant gene and replace it with a functional gene, potentially providing a permanent solution with a single administration. This fundamental therapeutic approach to disease management is called “silence and replace.” The silence and replace mechanism offers the potential to restore the normative physiology of diseased cells and tissues and to improve treatment outcomes for patients suffering from the chronic, and potentially fatal, effects of OPMD. BB-301 has been granted Orphan Drug Designation from the EMA and Orphan Drug and Fast Track Designations from the FDA.
About Benitec Biopharma, Inc.
Benitec Biopharma Inc. (“Benitec” or the “Company”) is a clinical-stage biotechnology company focused on the advancement of novel genetic medicines with headquarters in Hayward, California. The proprietary “Silence and Replace” DNA-directed RNA interference platform combines RNA interference, or RNAi, with gene therapy to create medicines that simultaneously facilitate sustained silencing of disease-causing genes and concomitant delivery of wildtype replacement genes following a single administration of the therapeutic construct. The Company is developing Silence and Replace-based therapeutics for chronic and life-threatening human conditions including Oculopharyngeal Muscular Dystrophy (OPMD). A comprehensive overview of the Company can be found on Benitec’s website at www.benitec.com.
Forward Looking Statements
Except for the historical information set forth herein, the matters set forth in this press release include forward-looking statements, including statements regarding Benitec’s plans to develop and commercialize its product candidates, the timing of the completion of pre-clinical and clinical trials, the timing of the availability of data from our clinical trials, the timing and sufficiency of patient enrollment and dosing in clinical trials, the timing of expected regulatory filings and other regulatory steps, and the clinical utility and potential attributes and benefits of ddRNAi and Benitec’s product candidates, and other forward-looking statements.
These forward-looking statements are based on the Company’s current expectations and subject to risks and uncertainties that may cause actual results to differ materially, including unanticipated developments in and risks related to: the success of our plans to develop and potentially commercialize our product candidates; the timing of the completion of preclinical studies and clinical trials; the timing and sufficiency of patient enrollment and dosing in any future clinical trials; the timing of the availability of data from our clinical trials; the timing and outcome of regulatory filings and approvals; the development of novel AAV vectors; our potential future out-licenses and collaborations; the plans of licensees of our technology; the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; our intellectual property position and the duration of our patent portfolio; expenses, ongoing losses, future revenue, capital needs and needs for additional financing, and our ability to access additional financing given market conditions and other factors; the length of time over which we expect our cash and cash equivalents to be sufficient to execute on our business plan; unanticipated delays; further research and development and the results of clinical trials possibly being unsuccessful or insufficient to meet applicable regulatory standards or warrant continued development; the ability to enroll sufficient numbers of subjects in clinical trials; determinations made by the FDA and other governmental authorities and other regulatory developments; the Company’s ability to protect and enforce its patents and other intellectual property rights; the Company’s dependence on its relationships with its collaboration partners and other third parties; the efficacy or safety of the Company’s products and the products of the Company’s collaboration partners; the acceptance of the Company’s products and the products of the Company’s collaboration partners in the marketplace; market competition; sales, marketing, manufacturing and distribution requirements; greater than expected expenses; expenses relating to litigation or strategic activities; the impact of, and our ability to remediate, the identified material weakness in our internal controls over financial reporting; the impact of local, regional, and national and international economic conditions and events; and other risks detailed from time to time in the Company’s reports filed with the Securities and Exchange Commission. The Company disclaims any intent or obligation to update these forward-looking statements.
Investor Relations Contact:
Irina Koffler
LifeSci Advisors, LLC
(917) 734-7387
ikoffler@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/8aeb15e8-1b57-4093-98b6-8d84522b188c
