New York, USA, Dec. 15, 2025 (GLOBE NEWSWIRE) -- Global Interventional Cardiology Devices Market is Predicted to Cross ~USD 30 Billion by 2034, Due to the Rising Cardiovascular Disease Burden | DelveInsight
The global market for interventional cardiology devices is being propelled by the rising incidence of cardiovascular diseases and related risk factors, the growing adoption of minimally invasive procedures, ongoing technological advancements in device design, and a surge in strategic initiatives by major industry players.
DelveInsight’s Interventional Cardiology Devices Market Insights report provides the current and forecast market analysis, individual leading interventional cardiology devices companies’ market shares, challenges, interventional cardiology devices market drivers, barriers, trends, and key interventional cardiology devices companies in the market.
Interventional Cardiology Devices Market Summary
- The global interventional cardiology devices market size is expected to increase from ~USD 20 billion in 2024 to ~USD 35 billion by 2032, reflecting strong and sustained growth.
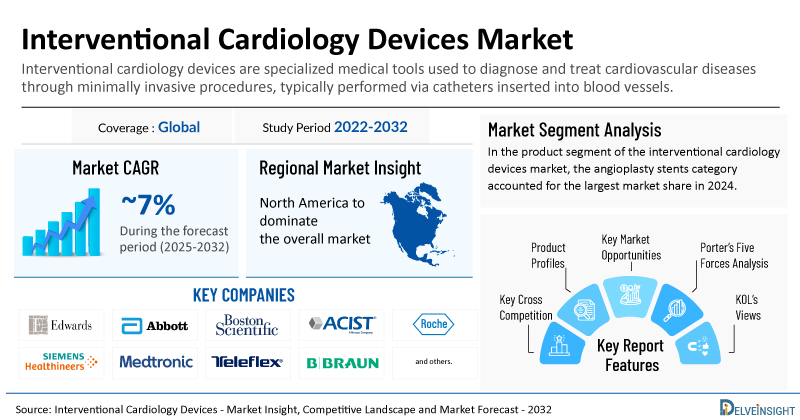
- The global interventional cardiology devices market is expected to grow at a CAGR of ~7% during the forecast period from 2025 to 2032.
- The leading companies working in the interventional cardiology devices market include Edwards Lifesciences Corporation, Abbott Laboratories, Boston Scientific Corporation, ACIST Medical Systems, F. Hoffmann-La Roche Ltd., Siemens Healthineers AG, Medtronic, Teleflex Incorporated, B. Braun SE, MicroPort Scientific Corporation, SIS MEDICAL AG, Lepu Medical Technology (Beijing) Co., Ltd., Terumo Corporation, Sahajanand Laser Technology Limited, Biotronik SE & Co. KG, Cordis, Abiomed, Inc., Merit Medical Systems, Koninklijke Philips N.V., Cook, and others.
- Among all the regions, North America is anticipated to register the fastest growth in the interventional cardiology devices market during the forecast period.
- In the product segment of the interventional cardiology devices market, the angioplasty stents category accounted for the largest market share in 2024.
To read more about the latest highlights related to the interventional cardiology devices market, get a snapshot of the key highlights entailed in the Global Interventional Cardiology Devices Market Forecast Report
Key Factors Contributing to the Rise in Growth of the Interventional Cardiology Devices Market
Growing burden of cardiovascular diseases
Increasing cases of heart-related conditions, fueled by risk factors like obesity, high blood pressure, diabetes, and sedentary habits, are driving the need for advanced interventional cardiology solutions. As disorders such as coronary artery disease and myocardial infarctions become more widespread, the use of stents, catheters, and minimally invasive cardiac procedures is expanding rapidly to support effective treatment and enhance patient outcomes.
The rising preference for minimally invasive cardiac procedures
These techniques provide advantages such as shorter hospitalization, faster recovery, fewer complications, and greater patient comfort compared to conventional open-heart surgeries. Continuous innovations in stents, catheters, and image-guided systems are enhancing both safety and effectiveness, encouraging broader adoption among patients and clinicians. As healthcare increasingly shifts toward less invasive treatments, global demand for interventional cardiology devices is accelerating significantly.
Rising innovation in device design
Ongoing technological advancements in the design of interventional cardiology devices are accelerating market growth by enhancing safety, accuracy, and clinical outcomes. Breakthroughs, such as bioresorbable and drug-eluting stents, next-generation balloon catheters, atherectomy tools, and AI-enabled imaging systems, are enhancing procedural efficiency and reducing complication risks. These advanced product designs expand the possibilities for minimally invasive care, facilitating faster adoption among clinicians and contributing to overall market growth.
Get a sneak peek at the interventional cardiology devices market dynamics @ Interventional Cardiology Devices Market Trends
Regional Interventional Cardiology Devices Market Insights
North America dominated the global interventional cardiology devices market in 2024 with an estimated 43% share. This leadership is supported by the region’s high incidence of cardiovascular diseases, well-established healthcare systems, and strong uptake of minimally invasive cardiac procedures. The presence of leading medical device companies, ongoing innovations—including drug-eluting stents, bioresorbable scaffolds, and catheter-based technologies—and substantial R&D investments further reinforce market growth.
Europe continues to play a significant role in advancing the cardiac monitoring and interventional cardiology devices sector, driven by the growing prevalence of cardiovascular conditions, an aging demographic, and a strong focus on preventive care. The region’s sophisticated healthcare infrastructure and government support for early diagnosis, product approvals, and the introduction of new technologies are also accelerating device adoption. For example, in October 2024, Medtronic secured CE Mark approval for its Evolut™ FX+ TAVI System, designed to offer improved coronary access and procedural efficiency. These factors collectively strengthen Europe’s overall market outlook.
The Asia-Pacific region is rapidly becoming a key growth engine for the interventional cardiology devices market, driven by rising cardiovascular disease rates, increasing prevalence of obesity and diabetes, and a large aging population. Expanding healthcare investments, modernization of hospital infrastructure, and growing availability of minimally invasive cardiac procedures are further boosting uptake. Government initiatives and the presence of cost-efficient device manufacturers are adding additional momentum.
To know more about why North America is leading the market growth in the interventional cardiology devices market, get a snapshot of the Interventional Cardiology Devices Market Share
Recent Developmental Activities in the Interventional Cardiology Devices Market
- In May 2025, iVascular and Medico’s Hirata announced that Luminor Drug Coated Balloon (DCB) has received approval from Japan’s Ministry of Health, Labour and Welfare (MHLW).
- In April 2025, Elixir Medical LithiX™ HC-IVL System was launched in Europe (date unspecified, but recent). This intravascular lithotripsy device targets calcified coronary lesions, expanding plaque-modification toolsets beyond traditional balloons and stents.
- In February 2025, Medtronic began enrolling patients in a new global clinical trial evaluating its Prevail paclitaxel-coated balloon catheter for use in percutaneous coronary intervention (PCI). The study is designed to assess the device’s effectiveness in treating in-stent restenosis (ISR) and de novo small vessel disease in patients with coronary artery disease (CAD).
What are Interventional Cardiology Devices?
Interventional cardiology devices are specialized medical tools used to diagnose and treat cardiovascular diseases through minimally invasive procedures, typically performed via catheters inserted into blood vessels. These devices enable cardiologists to open blocked arteries, repair structural heart defects, restore blood flow, and support heart function without the need for open-heart surgery. Common interventional cardiology devices include stents, balloons, catheters, guidewires, embolic protection systems, and closure devices. By reducing surgical trauma, shortening recovery time, and improving patient outcomes, these technologies play a critical role in modern cardiac care.
| Interventional Cardiology Devices Market Report Metrics | Details |
| Coverage | Global |
| Study Period | 2022–2032 |
| Interventional Cardiology Devices Market CAGR | ~7% |
| Interventional Cardiology Devices Market Size by 2032 | ~USD 35 Billion |
| Key Interventional Cardiology Devices Companies | Edwards Lifesciences Corporation, Abbott Laboratories, Boston Scientific Corporation, ACIST Medical Systems, F. Hoffmann-La Roche Ltd., Siemens Healthineers AG, Medtronic, Teleflex Incorporated, B. Braun SE, MicroPort Scientific Corporation, SIS MEDICAL AG, Lepu Medical Technology (Beijing) Co., Ltd., Terumo Corporation, Sahajanand Laser Technology Limited, Biotronik SE & Co. KG, Cordis, Abiomed, Inc., Merit Medical Systems, Koninklijke Philips N.V., Cook, and others |
Interventional Cardiology Devices Market Assessment
- Interventional Cardiology Devices Market Segmentation
- Interventional Cardiology Devices Market Segmentation By Product: Angioplasty Balloons [Drug-Eluting Balloons and Others], Angioplasty Stents [Drug-Eluting Stents, Bare-Metal Stents, Bioabsorbable Stents], Structural Heart Devices [Aortic Valve Therapy Devices and Others], Catheters [Angiography Catheters, Guiding Catheters, and IVUS/OCT Catheters], Plaque Modification Devices [Atherectomy Devices and Thrombectomy Devices], Hemodynamic Flow Alteration Devices, [Embolic Protection Devices and Chronic Total Occlusion Devices], and Others
- Interventional Cardiology Devices Market Segmentation By End-User: Hospitals, Ambulatory Surgical Centers, Others
- Interventional Cardiology Devices Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of World
- Porter’s Five Forces Analysis, Product Profiles, Case Studies, KOL’s Views, Analyst’s View
Which MedTech key players in the interventional cardiology devices market are set to emerge as the trendsetter explore @ Interventional Cardiology Devices Market Analysis
Table of Contents
| 1 | Interventional Cardiology Devices Market Report Introduction |
| 2 | Interventional Cardiology Devices Market Executive Summary |
| 3 | Competitive Landscape |
| 4 | Regulatory Analysis |
| 5 | Interventional Cardiology Devices Market Key Factors Analysis |
| 6 | Interventional Cardiology Devices Market Porter’s Five Forces Analysis |
| 7 | Interventional Cardiology Devices Market Layout |
| 8 | Interventional Cardiology Devices Market Company and Product Profiles |
| 9 | KOL Views |
| 10 | Project Approach |
| 11 | About DelveInsight |
| 12 | Disclaimer & Contact Us |
Interested in knowing the interventional cardiology devices market share by 2032? Click to get a snapshot of the Interventional Cardiology Devices Market Size
Related Reports
Angioplasty Balloons and Stents Market
Angioplasty Balloons and Stents Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key angioplasty balloons and stents companies, including B. Braun SE, BIOTRONIK, Medtronic, Abbott, Boston Scientific Corporation, Cook, Biosensors International Group, Ltd., MicroPort Scientific Corporation, Terumo Corporation, Demax Medical Technology, BD, BrosMed Medical Co., Ltd., Cordis, Sino Medical Sciences Technology Inc., Concept Medical, among others.
Drug-Eluting Balloons Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key drug-eluting balloons companies, including Boston Scientific Corporation, BD, Terumo Corporation, Medtronic, Koninklijke Philips N.V., M.A. Med Alliance SA., Cook, BIOTRONIK, SurModics Inc., B. Braun SE, Aachen Resonance Entwicklungsgesellschaft mbH, Biosensors International Group, Ltd., Eurocor GmbH, iVascular, Innvolution, Cardiovascular Systems, Inc., Genesis Medtech, Concept Medical, Cardionovum GmbH, YINYI (LIAONING) BIOTECH CO., LTD., among others.
Drug-Eluting Stents Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key drug-eluting stents companies, including Medtronic, Abbott, Boston Scientific Corporation, B. Braun Melsungen AG, BIOTRONIK SE & Co. KG, Cook, Terumo Corporation, Lepu Medical Technology Co., Ltd., Microport Scientific Corporation, Balton Sp.z.o.o, ENDOCOR GmbH, Eurocor Tech GmbH, Alvimedica, Biosensors International Group, Ltd., Meril Life Sciences Pvt. Ltd., Translumina, Sino Medical Sciences Technology Inc., iVascular S.L.U, Sahajanand Laser Technology Limited, Cardionovum GmbH, among others.
Structural Heart Devices Market
Structural Heart Devices Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key structural heart devices companies, including Medtronic, Boston Scientific Corporation, Braile Biomedica, JOTEC GmbH, Edwards Life Sciences Corporation, Valcare Medical, W. L. Gore & Associates, Inc., Abbott Laboratories, Cardiovascular Systems, Inc., XELTIS BV, JenaValve Technology, Inc., Biomerics, Lepu Medical Technology (Beijing) Co., Ltd., LivaNova Inc., Comed B.V., TORAY, INDUSTRIES INC., Occlutech International AB, Microport Scientific Corporation, Meril Life Sciences Venus Medtech (Hangzhou) Inc., among others.
Atherectomy Devices Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key atherectomy devices companies, including Koninklijke Philips N.V., Medtronic PLC, Boston Scientific Corporation, AngioDynamics Inc., Abbott Laboratories, Avinger, BD, Ra Medical Systems, REX Medical, Cardio Flow, Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
