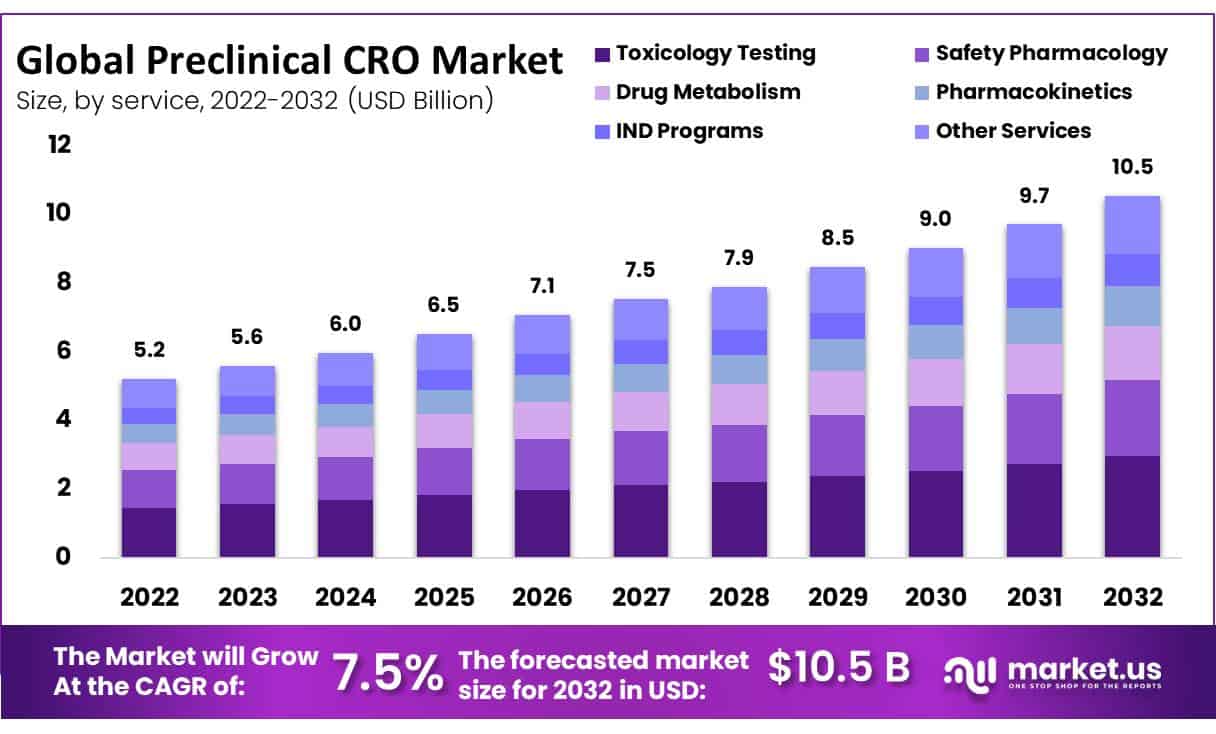
New York, Oct. 19, 2023 (GLOBE NEWSWIRE) -- The Preclinical CRO Market size is expected to be worth around USD 10.5 Billion by 2032 from USD 5.2 Billion in 2022, growing at a CAGR of 7.5% during the forecast period from 2023 to 2032.
This market is projected to grow at a high compound annual growth rate of 7.5% between 2023 and 2032. The method used for the production of drugs and medical devices is extremely complex, requiring massive levels of capital and assets to achieve an optimal result. CROs mitigate the costs of creating products for a specialty market by reducing organizations' expenses. Furthermore, they are designed to assist the introduction process of medicines and devices by providing advice from industry experts as well as helping them in conducting preliminary studies.
Key Takeaway
- By Services, toxicology testing remained the largest contributor to the preclinical CRO market in 2022.
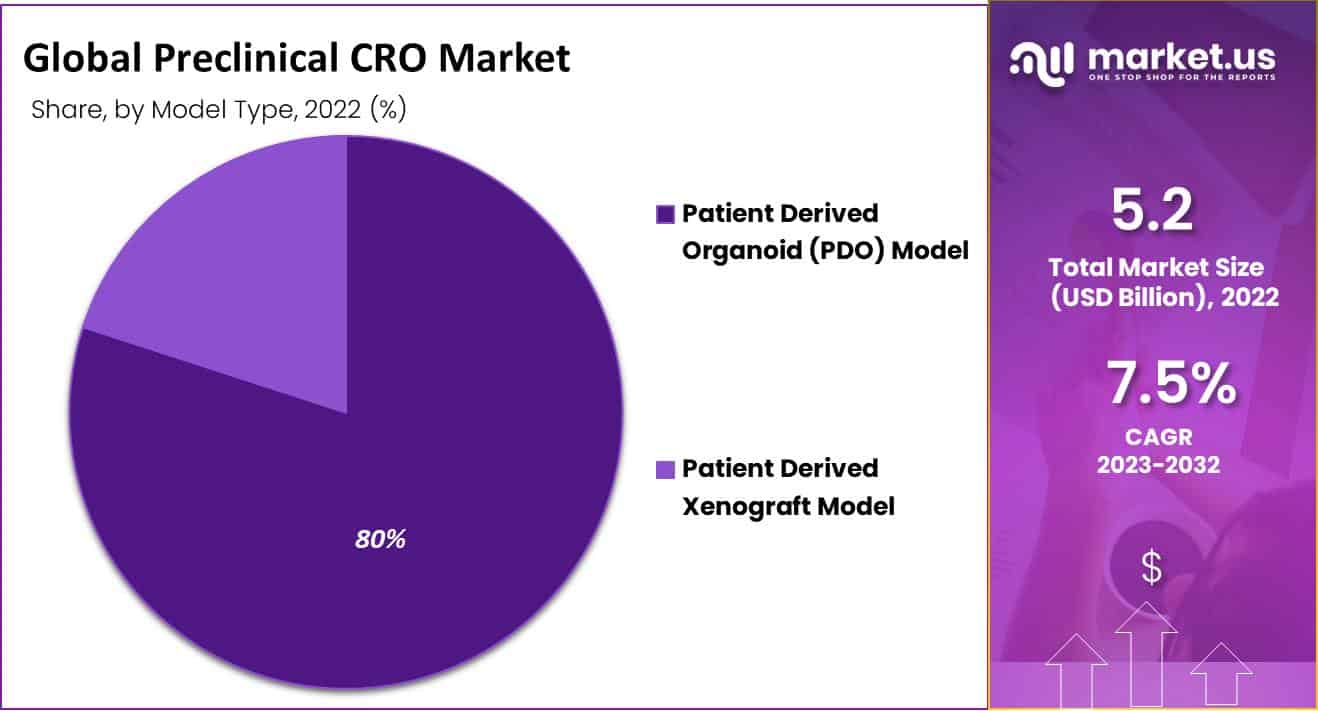
- By Model Type, Patient-Determined Organoid (PDO) Model segment held the largest market share, amounting to 80%.
- By end user, the Pharmaceutical and Biopharmaceutical companies were top contributors to the market in 2022.
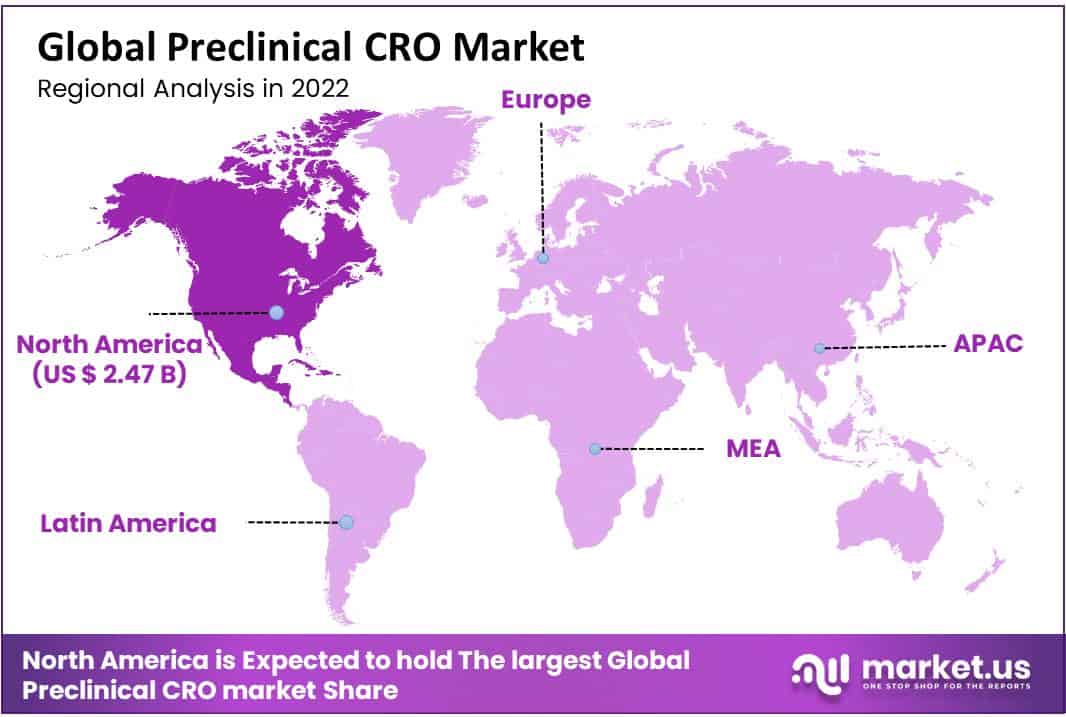
- North America generated the most revenue region wise, with a market share of 47.50%
- Asia Pacific is expected to undergo a period of exponential growth throughout the forecast.
Market.us has identified key trends, drivers, and challenges in the market, which will help clients improve their strategies to stay ahead of their competitors. - View a PDF sample report @ https://market.us/report/preclinical-cro-market/request-sample/
Factors affecting the growth of the Preclinical CRO Market
- One of the main factors that influence the growth of the Preclinical CRO market is the increase in subcontracting of non-core function. With this, the market is also being propelled towards expansion.
- Another factor that impacts the market is the unavailability of skilled personnel. The demand for professionals with acumen is high, but the supply is disproportionate.
- Further, the market is impacted by use of advanced technologies. By utilizing technology, the burden on human employees can be lowered. Further, doing so introduces efficiency of the functions of the organisation, leading to enhanced productivity.
Top Trends in the Global Preclinical CRO Market
There has been increase in outsourcing for preclinical CRO by firms specialising in medical devices. A preclinical CRO is cost-effective while being efficient as well. By using the services of a CRO a pharmaceutical business can effectively carry out research with faster turnaround. This has enabled the market to grow further.
Additionally, another trend observed is collaborations between clinical CRO and pharmaceutical businesses. For example, the IRBM entered into a partnership with Merck & co. and signed an agreement to continue said partnership in July 2023. This enables pharmaceutical businesses to launch their drugs into the market at a much faster rate.
Market Growth
With the increasing number of drugs in development, the preclinical CRO market is expected to undergo exponential growth. With the variety of benefits provided by preclinical CROs, such as flexibility, administrative assistance, regulatory compliance and time-effectiveness, the market for preclinical CRO is sure to experience accelerated growth.
Regional Analysis
North America accounted for the largest share at 47.50% in 2022, driven by the presence of CROs with prior drug discovery expertise, such as Charles Waterway Research and LabCorp. The United States is the largest market for outsourcing preclinical trials, this is mainly attributed to the desire to conduct trials in accordance with the regulations pf the FDA. Such businesses are supported by Investigational New Drug (IND) Application, which has garnered the approval of the FDA.
Asia Pacific is also expected to expand at the highest rate of 10.9% throughout the forecast period. The increasing usage of services of CROs can be credited to the changing landscape of the market, which has led to an increase in funds allocated to the research and development. Furthermore, developing economies such as China and India show propensity towards government spending, which also drives market growth. Companies deployed in Europe and the United States are looking to streamlined regulatory bodies, on-site research advancements, and clinical operations in the Asia-Pacific region to reduce costs associated with research.
To understand how our report can bring a difference to your business strategy, Inquire about a brochure at https://market.us/report/preclinical-cro-market/#inquiry
Scope of the Report
| Report Attributes | Details |
| Market Value (2022) | USD 5.2 Billion |
| Market Size in 2032 | USD 10.5 Billion |
| CAGR (2023 to 2032) | 7.5% |
| North America Revenue Share | 47.50% |
| Historic Period | 2016 to 2021 |
| Base Year | 2022 |
| Forecast Year | 2023 to 2032 |
Market Drivers
The trend of continual revaluating denotes one of the key variables influencing market development. In addition, preclinical CROs also provide end-to-end regulatory services, such as toxicology testing, which also contributes to market growth. Other drivers includes minimal equipment costs and the increasing prevalence of persistent infections that are expected to drive growth.
Market Restraints
The market growth is likely to be hindered due to lack of trained personnel in the CRO firmament. Seeing that CRO operates in the research and development domain, it becomes imperative for the personnel to possess detailed knowledge and polished skills. However, there is a deficit of skilled labor. Also, the cost of labor is considerably high in the CRO market. This potentially presents challenges for market growth.
Market Opportunities
Firms specialising in medical devices are likely to outsource testing procedures. This presents opportunities for firms operating in the preclinical CRO market. By outsourcing, the medical devices businesses will reduce the strain on their operations. On the other hand, CRO firms will be presented with opportunities to generate more revenue and expand their market shares.
Immediate Delivery Available | Buy This Premium Research Report @ https://market.us/purchase-report/?report_id=104628
Report Segmentation of the Preclinical CRO Market
Service Insight
The global market for preclinical CROs is dominated by toxicology testing. In particular, toxicology tests are vital to detect novel and newly developed medicines before they are tested on humans. By doing so, researchers are not only testing the tolerability and efficacy of this medicine but also describing possible toxic effects that it may have.
Model Type Insight
The patient-determined organ (PDO) model accounted for an unmatched largest market share amounting to 80.47% in 2022. The growing impact of the PDO model is a result of the use of cells and tissues obtained directly from the patient. This allows health services to be reworked and models to be preserved. They, therefore become an important element of preclinical evaluation because they facilitate faster termination and predict harm.
End-User Insight
Pharmaceutical and Biopharmaceutical organizations contributed the most to the preclinical CRO market in 2022. This can be attributed to their expansive reach and availability of funds. Alternatively, academic research organizations are expected to register a CAGR of 8.2% throughout the forecast period.
Market Segmentation
By Service
- Toxicology Testing
- Safety Pharmacology
- Drug Metabolism
- Pharmacokinetics
- IND Programs
- Other Services
By Model Type
- Patient Derived Organoid (PDO) Model
- Patient Derived Xenograft Model
By End-User
- Pharmaceutical and Biopharmaceutical companies
- Medical Device manufacturing companies
- Academic Research Organizations
- Other End Users
By Geography
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Market.us has identified key trends, drivers, and challenges in the market, which will help clients improve their strategies to stay ahead of their competitors. - View a PDF sample report @ https://market.us/report/preclinical-cro-market/request-sample/
Competitive Landscape
The competition in the preclinical CRO market is intense with firms looking to cement their positon in the market or grow their respective market shares. To ensure high revenue, businesses employ tactics like partnerships, mergers and acquisitions. The worldwide market for Preclinical CRO is characterised by several prominent players such as PAREXEL International Corporation, Laboratory Corporation of America Holdings, Medpace, Inc., Envigo Corporation, Charles River Laboratories, PRA Health Sciences, Inc., PPD Inc., and Covance Inc.
Market Key Players
- PAREXEL International Corporation
- Laboratory Corporation of America Holdings
- Medpace, Inc.
- Envigo Corporation
- Charles River Labs
- PRA Health Science, Inc.
- PPD Inc.
- Covance Inc.
- Other Key Players.
Recent Development of the Preclinical CRO Market
- In April 2023, Proscia announced the deployment of Concentriq for Research by Virscio. This is a CRO firm that will utilise cloud platform developed by Proscia to introduce efficiency in the process of drug delivery by providing valuable insights.
- In October 2023, LabCorp introduced Amyloid-Tau-Neurodegeneration Profile, a blood test that diagnose Alzheimer’s Diseases. While the test is not precise in its diagnosis, it can inform physicians regarding the possibility of developing said disease.
Browse More Related Reports
- Healthcare Chatbots market size was valued at USD 195.85 million and is expected to reach USD 1168 million in 2032
- Addiction Rehab Facilities Market accounted for USD 15.6 billion and is expected to grow to around USD 31.3 billion in 2032
- Health And Wellness Market size is expected to be worth around USD 4,332 Billion by 2023 from USD 8,379 Billion in 2032
- Antibody Drug Conjugates Market accounted for USD 7.2 billion and is expected to grow to around USD 34.7 billion in 2032
- Telemedicine Market is expected to be worth around USD 590.9 Bn by 2032 from USD 63.5 Bn in 2022
- Women’s Health Rehabilitation Products Market size is expected to be worth around USD 7.7 Billion by 2032 from USD 4.5 Billion in 2022
- Telehealth Market was valued at USD 7.7 Billion. Between 2023 and 2032
- Medical Devices Market size is expected to be worth around USD 656 Mn by 2032 from USD 492 Mn in 2022
- Artificial Insemination Market size is expected to be worth around USD 4,784 Million by 2032 from USD 2,140 Million in 2022
- Clinical Trial Imaging Market was valued at US$ 1,067.3 Million. Between 2023 and 2032
- Biomaterials Market size is expected to be worth around USD 540.5 Billion by 2032 from USD 155.9 Billion in 2022
- Predictive & Personalized Medicine Market size is expected to be worth around USD 692.0 Billion by 2032 from USD 347.2 Billion in 2023
- Knee Replacement Market size is expected to be worth around USD 16.6 Billion by 2032 from USD 10.3 Billion in 2022
About Us:
Market.US (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. Market.US provides customization to suit any specific or unique requirement and tailor-makes reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us on LinkedIn | Facebook | Twitter
Our Blog:
