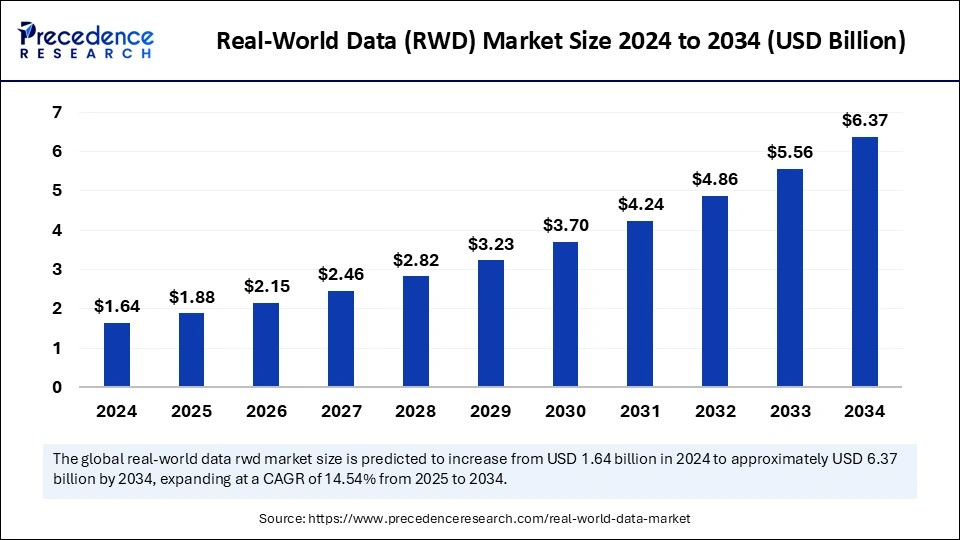
Ottawa, Dec. 09, 2025 (GLOBE NEWSWIRE) -- The global real-world data (RWD) market size is expected to be worth over USD 6.37 billion by 2034, increasing from USD 2.15 billion in 2026, growing at a strong CAGR of 14.54% between 2025 and 2034. The shift towards personalised medicine and growing drug development drives the market growth.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/5694
Real-World Data (RWD) Market Highlights:
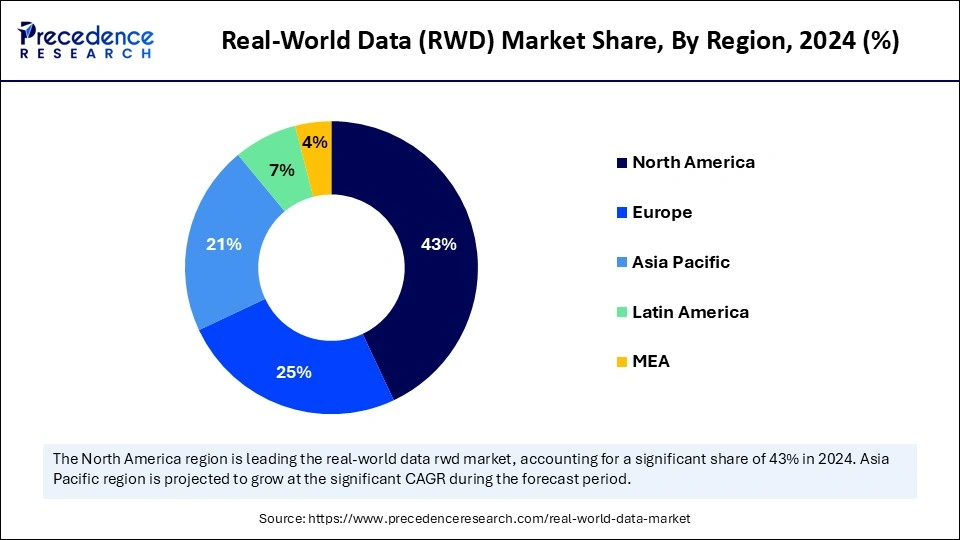
- North America dominated the market with 43% share in 2024, driven by advanced healthcare systems and strong adoption of RWE.
- Asia Pacific is set to grow at the fastest pace (CAGR ~11%), supported by digital health expansion and rising clinical trials.
- Services led the component segment in 2024, reflecting high demand for analytics, consulting, and regulatory support.
- Datasets will be the fastest-growing component, boosted by increasing EHR usage, wearables, and real-time health data generation.
- Drug Development & Approvals was the leading application, as RWD accelerates trials and supports regulatory submissions.
- Post-Market Surveillance is projected to see the highest CAGR, driven by greater emphasis on long-term safety and effectiveness monitoring.
- Pharmaceutical & Medical Device Companies held the largest end-user share in 2024, fueled by strong R&D and precision medicine initiatives.
- Healthcare Providers will grow the fastest, adopting RWD for patient management, treatment optimization, and operational efficiency.
What is Real-World Data (RWD)?
The real-world data (RWD) market growth is driven by a strong emphasis on value-based care, focus on improving drug development, expansion of digital health records, high prevalence of chronic diseases, and rapid growth in digital health technologies.
Real-world data is observational data about healthcare delivery & patient health status that is derived from sources like insurance claims, wearable devices, electronic health records (EHRs), and patient registries. These data are available in unstructured or structured form. RWD generates Real-World Evidence and offers a comprehensive picture of the performance of medical products.
➡️ Become a valued research partner with us https://www.precedenceresearch.com/schedule-meeting
Private Industry Investments in the Real-World Data (RWD) Industry:
- Tempus: This AI and precision medicine company, which deals extensively in RWD from sources like lab data and genomics, has achieved a high private valuation, supported by its data quality and predictive analytics capabilities.
- Komodo Health: A private firm with a significant valuation, Komodo Health uses a comprehensive view of healthcare data to provide real-world insights through an extensive network of data partnerships.
- ConcertAI: This company focuses on using RWD and AI for life sciences and has a high valuation among private RWD providers, indicating substantial investment in its platform for accelerating clinical research and development.
- IQVIA: As a major global provider of advanced analytics, technology solutions, and contract research services, IQVIA is a key player in the RWD market, partly due to strategic initiatives and partnerships with companies like Salesforce.
- PPD, Inc. (now part of Thermo Fisher Scientific): PPD was acquired by Thermo Fisher Scientific, a significant private industry move that integrated its RWD and clinical research expertise into a larger scientific instrumentation and services company.
✚ Get informed with deep-dive intelligence on AI’s market impact https://www.precedenceresearch.com/ai-precedence
Real-World Data (RWD) Market Key Trends
- Growing adoption of AI and machine learning
AI-powered analytics are becoming essential for processing and analyzing the massive, complex datasets from real-world sources. This helps biopharma companies gain actionable insights more quickly and efficiently, accelerating drug development and informing commercial strategies.
- Expansion of RWD sources beyond HER
The market is moving beyond standard electronic health records (EHRs) to incorporate a wider variety of digital health technologies, such as data from wearables, mobile health apps, and patient-reported outcomes. This provides a richer, more continuous stream of data about patients' health behaviors and outcomes in everyday life.
- Emphasis on patient-centricity
Pharmaceutical companies are increasingly using RWD to understand patient experiences and incorporate their perspectives throughout the drug development process. This is leading to clinical trials that are more inclusive and representative of diverse patient populations, which helps address unmet needs.
- Increasing regulatory acceptance
Major regulatory agencies like the FDA and EMA are growing more receptive to the use of real-world evidence (RWE), which is derived from RWD, to support regulatory decisions and label expansions. This growing acceptance reduces reliance on traditional, expensive clinical trials, particularly in areas like rare diseases, by providing evidence from real-world performance.
➤ Get the Full Report @ https://www.precedenceresearch.com/real-world-data-rwd-market
Real-World Data (RWD) Market Opportunity
Growing Drug Development Unlocks RWD Market Opportunity
The growing demand for personalised medicine and the rising prevalence of diseases increase the demand for drug development. The strong focus on accelerating the drug development process and minimizing drug production time increases demand for RWD. The increasing need for predicting drug response requires RWD.
The strong focus on enhancing the safety of drugs and growing identification of novel therapeutic targets increases demand for RWD. The strong focus on understanding real-world treatment effectiveness and strong support for drug repurposing increase the adoption of RWD. The growing drug development creates an opportunity for the growth of the market.
Real-World Data (RWD) Market Report Coverage
| Report Coverage | Details | |
| Market Size (2025) | USD 1.88 Billion | |
| Market Size (2026) | USD 2.15 Billion | |
| Market Size (2034) | USD 6.37 Billion | |
| CAGR (2025–2034) | 14.54% | |
| Dominating Region | North America | |
| Fastest Growing Region | Asia Pacific | |
| Base Year | 2024 | |
| Forecast Period | 2025 to 2034 | |
| Segments Covered | Component, Application, End User, and Regions | |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa | |
| Key Components | Services (largest share), Datasets (fastest growing) | |
| Top Applications | Drug Development & Approvals; Post-Market Surveillance | |
| Major End Users | Pharmaceutical & Medical Device Companies; Healthcare Providers | |
| Key Market Drivers | Rise of evidence-based medicine, telehealth expansion, decentralized trials, growth in precision medicine, increased wearable/IoT health data, regulatory support for RWE | |
| Challenges | Data interoperability, lack of standardization across sources | |
| Opportunities | Increasing RWE use in approvals, demand for post-market safety data, value-based care adoption, chronic disease management insights | |
| Notable Market Players | IQVIA, Optum, Flatiron Health, IBM, SAS, Palantir, Tempus, Evidera | |
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/5694
Real-World Data (RWD) Market Regional Outlook
Why North America Dominates the Real-World Data (RWD) Market?
North America dominated the market with a 43% share in 2024. The strong presence of advanced healthcare infrastructure and high adoption of EHRs increases demand for RWD. The high prevalence of chronic diseases and a well-established pharmaceutical sector increase the adoption of RWD. The supportive government policies, like the U.S. FDA and a shift towards value-based care, drive the overall market growth.
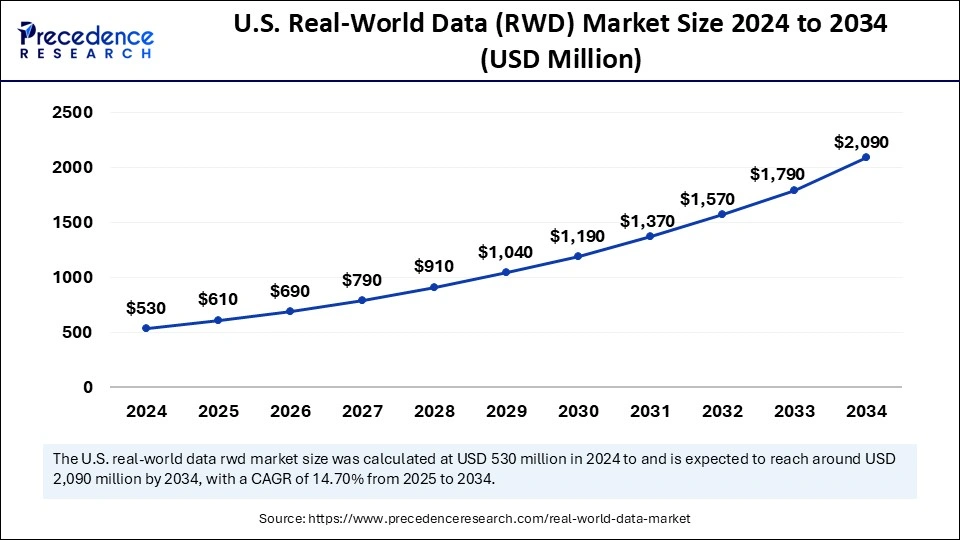
What is the U.S. Real-World Data (RWD) Market Size and Growth?
According to Precedence Research, the U.S. real-world data (rwd) market size is valued at USD 610 million in 2025 and is expected to surpass USD 2,090 million by 2034, with a noteworthy CAGR of 14.70% from 2025 to 2034.
U.S. Real-World Data (RWD) Market Trends
The U.S. market is expanding as healthcare systems, insurers, and biopharma companies increasingly use real-world evidence to optimize clinical outcomes and reduce costs. Growing integration of electronic health records, wearable devices, and claims data is enhancing the depth and accuracy of patient insights.
The shift towards value-based care is accelerating demand for advanced RWD analytics to support treatment effectiveness assessments and reimbursement decisions.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Try Before You Buy – Get the Sample Report@ https://www.precedenceresearch.com/sample/5694
How is the Asia Pacific experiencing the Fastest Growth in the Real-World Data (RWD) Market?
Asia Pacific is experiencing the fastest growth in the market during the forecast period. The growing prevalence of chronic diseases and increasing life expectancy increase demand for RWD. The strong government support for RWD use in countries like China & Japan, and increasing investment in analytics solutions & digital health, help market growth. The strong presence of clinical trial landscapes and technological innovations like cloud-based analytics, the rise of big data, & artificial intelligence supports the overall market growth.
China Real-World Data (RWD) Market Trends
China’s market is growing rapidly as healthcare providers and pharmaceutical companies increasingly rely on real-world evidence to support clinical decision-making and accelerate drug development. Expanded digital health infrastructure, including widespread electronic medical records and government-backed health data platforms, is enabling more efficient data collection and integration.
Real-World Data (RWD) Market Segmentation Insights
Component Insights
Why the Services Segment Held the Largest Share in the Real-World Data (RWD) Market?
The services segment held the largest revenue share of the market in 2024. The well-established pharmaceutical sector and growing development of drugs increase demand for services. The growing shift towards value-based care and a strong focus on product approvals increases the adoption of services. The rise in clinical trials and the growing development of drugs increases the adoption of services, supporting the overall market growth.
The datasets segment is the fastest-growing in the market during the forecast period. The growing adoption of wearable devices and the adoption of electronic health records increases the production of datasets. The strong focus on drug approvals and the shift towards personalised medicine increases demand for datasets. The growing adoption of digital transformation, like e-health services, the internet, and others, supports the overall market growth.
Application Insights
How Drug Development and Approvals Segment Dominated the Real-World Data (RWD) Market?
The drug development and approvals segment dominated the market in 2024. The regulatory agencies, like EMA and FDA, increase drug development and approvals. The growing development of traditional drugs and focus on optimizing clinical trial designs increases demand for RWD. The growing therapeutic area complexity in areas like rare disease & oncology increases adoption of RWD, driving the overall market growth.
The post-market surveillance segment is experiencing the fastest growth in the market during the forecast period. The strong focus on the detection of rare adverse reactions and long-term effects of drugs increases demand for post-market surveillance. The growing demand for increasing product effectiveness and enhancing safety features helps market growth. The availability of EHRs and the rise in digital health tools support the overall market growth.
End User Insights
Which End User Held the Largest Share in the Real-World Data (RWD) Market?
The pharmaceutical and medical device companies segment held the largest revenue share of the market in 2024. The growing population of patients and extensive clinical trials help market growth. The strong presence of regulatory bodies like the European Medicines Agency and the U.S. FDA, and growing drug development, helps in the expansion of pharmaceutical companies. The high R&D spending and shift towards value-based care support the overall market growth.
The healthcare providers segment is the fastest-growing in the market during the forecast period. The strong focus on patient management and the growing development of personalised medicines requires healthcare providers. The increasing need to lower drug development delays and monitor disease progression requires healthcare providers. The high prevalence of chronic diseases and a strong focus on enhancing operational efficiency support the overall market growth.
✚ Related Topics You May Find Useful:
➡️ Real-World Evidence (RWE) Solutions Market: Explore how data-driven decision-making is transforming clinical approvals and regulatory pathways
➡️ Life Science Big Data Market: Uncover how AI and analytics are accelerating breakthroughs in genomics, drug discovery, and precision medicine
➡️ Clinical Data Management Market: Track how digital platforms and automation are enhancing trial efficiency and data accuracy worldwide
➡️ Synthetic Control Arms Market: Learn how RWD-powered virtual controls are reducing trial costs and reshaping evidence generation
➡️ Healthcare Data Monetization Solutions Market: See how health organizations are unlocking new revenue streams through secure, compliant data utilization
➡️ Health Economics and Outcomes Research (HEOR) Services Market: Understand how HEOR insights guide market access, pricing strategies, and value-based healthcare decisions
➡️ DNA Data Storage Market: Discover how DNA-based storage is emerging as the next frontier for ultra-long-term, high-density data archiving
Top Companies in the Real-World Data (RWD) Market & Their Offerings:

- Cerner Corporation: Offers access to de-identified EHR data via a secure, cloud-based platform for research.
- Evidera, Inc.: Provides consulting and studies using RWD to demonstrate drug effectiveness, safety, and value to regulators.
- Flatiron Health, Inc.: Specializes in high-quality, curated oncology datasets from EHRs for cancer research.
- IBM Corporation: Uses AI and software platforms to analyze RWD, integrating client data for predictive analytics.
- IQVIA Holdings Inc.: Combines extensive healthcare datasets, advanced analytics, and services to accelerate drug development with RWE.
- Optum, Inc.: Utilizes vast insurance claims and pharmacy data to provide RWE solutions that improve healthcare system efficiency.
- Palantir Technologies Inc.: Offers a software platform to help organizations integrate and manage their own RWD sources for unified analysis.
- SAS Institute Inc.: Provides analytics and AI software enabling stakeholders to manage and analyze diverse RWD sources for decision-making.
- Syneos Health Inc.: Offers integrated RWE services, using a data ecosystem to design and execute real-world and late-phase clinical studies.
- Tempus Labs Inc.: Focuses on precision medicine by building one of the largest libraries of clinical and molecular data for research.
Recent Developments in the Real-World Data (RWD) Industry
- In February 2025, CitiusTech launched a real-world data platform, CitiusTech HealthSPARX, for life sciences organizations. The platform offers accelerated insights and efficient management of data. The platform supports research, commercial, clinical, and medical operations. (Source: https://www.biospace.com)
- In January 2025, Carelon Research launched new real-world data, Carelon Real World Data, to support healthcare evidence generation. The data includes enrolment records since 2006, enhanced oncology data, pharmacy & medical closed claims, commercial & medicare populations, and laboratory results. (Source: https://www.carelonresearch.com)
- In May 2024, HealthVerity collaborated with Castor to launch the platform, eConsent, to incorporate real-world data throughout the clinical trial lifecycle. The platform offers details about how patient RWD is used and is customizable. The platform contextualizes findings during the trial, conducts long-term follow-up, enhances eligibility screening, and continues monitoring of patients. (Source: https://www.castoredc.com)
Segments Covered in the Report
By Component
- Services
- Datasets
By Application
- Drug Development & Approvals
- Market Access & Reimbursement/Coverage Decisions
- Post-market Surveillance
- Clinical Research
- Other
By End User
- Pharmaceutical & Medical Device Companies
- Healthcare Payers
- Healthcare Providers
- Government Agencies
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Rest of North America
- South America
- Brazil
- Argentina
- Rest of South America
- Europe
- Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
- Western Europe
- Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
- MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
- GCC Countries
Thank you for reading. You can also get individual chapter-wise sections or region-wise report versions, such as North America, Europe, or Asia Pacific.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/5694
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Towards Packaging | Towards Automotive | Towards Chem and Materials | Towards FnB | Towards Consumer Goods | Statifacts | Towards EV Solutions | Towards Dental | Nova One Advisor | Market Stats Insight | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Get Recent News:
https://www.precedenceresearch.com/news
For the Latest Update Follow Us:
LinkedIn | Medium | Facebook | Twitter
